Exploring Gliclazide's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Gliclazide's R&D Progress
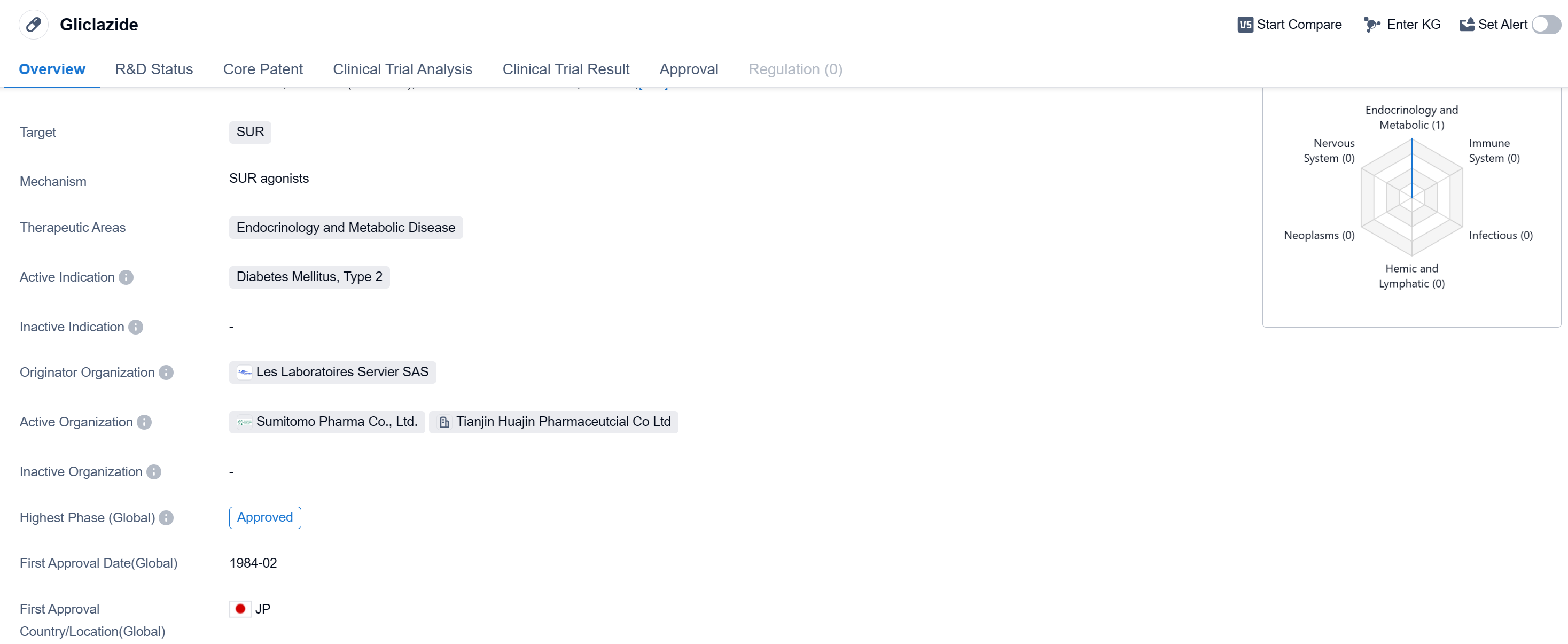
Gliclazide is a small molecule drug that falls under the therapeutic area of endocrinology and metabolic disease. It specifically targets the SUR (sulfonylurea receptor) protein. The drug is primarily used for the treatment of Diabetes Mellitus, Type 2.
Gliclazide was first approved in Japan in February 1984, making it one of the earliest drugs available for the treatment of Type 2 diabetes. The drug is manufactured by Les Laboratoires Servier SAS, a pharmaceutical company that serves as the originator organization for Gliclazide.
Gliclazide has achieved the highest phase of approval in the global market. The drug has been approved for use in treating Type 2 diabetes in both regions.
As a small molecule drug, Gliclazide works by targeting the SUR protein. This protein is involved in the regulation of insulin secretion in the pancreas. By targeting SUR, Gliclazide helps to increase insulin production and improve glucose control in patients with Type 2 diabetes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Gliclazide: SUR agonists
SUR agonists refer to substances or compounds that activate or stimulate the SUR (sulfonylurea receptor) protein. The SUR protein is a subunit of ATP-sensitive potassium (KATP) channels found in various tissues, including pancreatic beta cells, cardiac muscle cells, and smooth muscle cells. These channels play a crucial role in regulating insulin secretion, cardiac function, and smooth muscle relaxation.
By binding to the SUR protein, SUR agonists enhance the activity of KATP channels, leading to the opening of these channels. This opening results in the efflux of potassium ions from the cell, leading to membrane hyperpolarization and subsequent inhibition of calcium influx. Ultimately, this leads to various physiological effects depending on the tissue involved.
In the context of biomedicine, SUR agonists can have therapeutic implications. For example, in the treatment of type 2 diabetes, SUR agonists like sulfonylurea drugs (e.g., glibenclamide, glimepiride) are commonly used to stimulate insulin release from pancreatic beta cells. These drugs bind to the SUR protein, leading to KATP channel opening, depolarization of the cell membrane, and subsequent insulin secretion.
Furthermore, SUR agonists have also been investigated for their potential cardiovascular benefits. By activating KATP channels in cardiac muscle cells, these agonists can induce cardioprotective effects, such as reducing ischemic injury during myocardial infarction.
Overall, SUR agonists are substances that activate the SUR protein, leading to the modulation of KATP channels and subsequent physiological effects.
Drug Target R&D Trends for Gliclazide
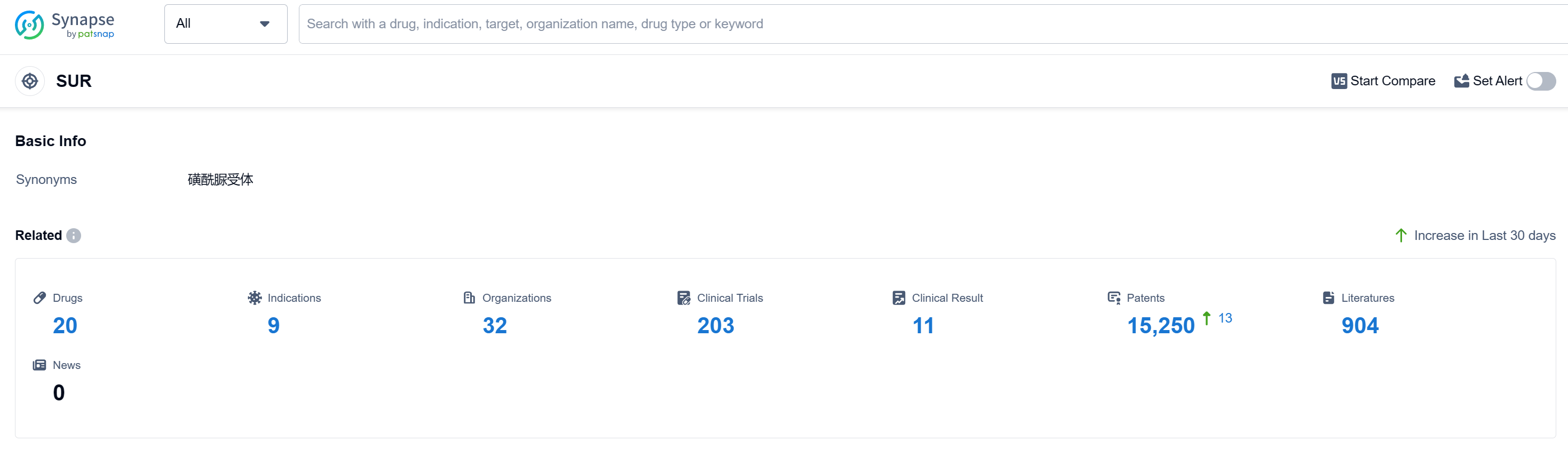
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 20 SUR drugs worldwide, from 32 organizations, covering 9 indications, and conducting 203 clinical trials.
Based on the analysis of the provided data, the current competitive landscape for target SUR in the pharmaceutical industry is characterized by the presence of multiple companies actively developing drugs for this target. Pfizer Inc., GSK Plc, Sanofi, and several other companies have successfully developed drugs for target SUR and obtained regulatory approval. The primary focus of research and development is on small molecule drugs, with a significant number of drugs in the approved phase. The indications for the approved drugs primarily revolve around diabetes-related conditions. China, Japan, and the United States are the leading countries/locations in terms of drug development for target SUR, with China having the highest number of drugs in the approved phase. Overall, the future development of target SUR is promising, with ongoing research and development activities and a competitive landscape driven by multiple companies and countries/locations.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Gliclazide is a small molecule drug developed by Les Laboratoires Servier SAS for the treatment of Type 2 diabetes. It targets the SUR protein and has achieved the highest phase of approval in the global markets. With its first approval in Japan in 1984, Gliclazide has a long history of use in managing diabetes and continues to be an important therapeutic option for patients worldwide.