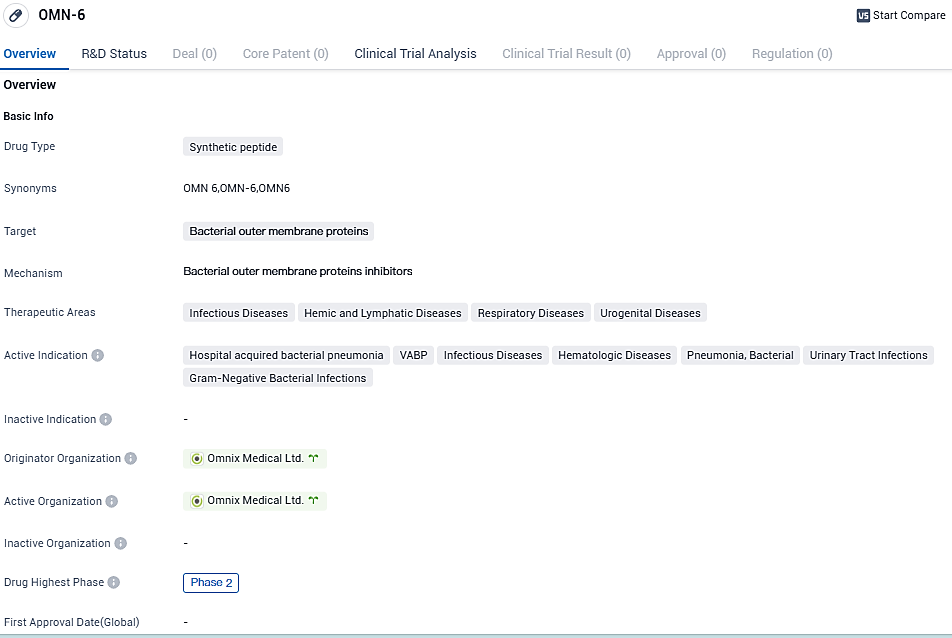
Omnix Medical gets green light from U.S. FDA for Phase II testing of its advanced anti-infective agent OMN6
The biopharmaceutical company Omnix Medical, known for creating advanced anti-infectives to combat serious infections, has reported today that their upcoming Phase II trial for their innovative anti-infective, OMN6, has received approval from the FDA.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The global, multi-site study aims to evaluate the safety and pharmacokinetics of OMN6 in patients suffering from either hospital-acquired bacterial pneumonia or ventilator-associated bacterial pneumonia induced by Acinetobacter baumannii complex.
OMN6, a breakthrough, pioneer antimicrobial peptide originated from insect host defense peptides, is the primary compound of Omnix Medical. Its working principles contrast starkly with traditional anti-infectives; it destroys bacterial cell membranes, leading to swift and potent action, unaffected by bacterial genotype or resistance phenotype.
During a randomized, double-blind Phase I study with a placebo-controlled, single ascending dose, and more than 80 healthy volunteers, OMN6 showcased an exceptional safety profile and tolerability at clinically valuable dosage levels. Furthermore, total clearance of the pharmaceutical was achieved, facilitating multiple daily infusions typical in anti-infective treatment regimes.
“We are thrilled with the U.S. FDA’s approval of our proposed Phase II study,” expressed Dr. Moshik Cohen-Kutner, Omnix Medical’s CEO. “Given the promising outcomes we’ve observed in our prior Phase I trial, we anticipate the Phase II outcomes will corroborate these findings. This could signify a crucial step forward in the creation of a new anti-infectives category, which doesn’t induce antimicrobial resistance.”
According to Dr. Niv Bachnoff, Omnix Medical’s CSO, “Our intention is to kickstart the Phase II trial in the approaching months. Our ultimate aim is to introduce a powerful alternative to traditional anti-infectives, thereby saving the lives of millions of global patients who are currently resistant to available antibiotics.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
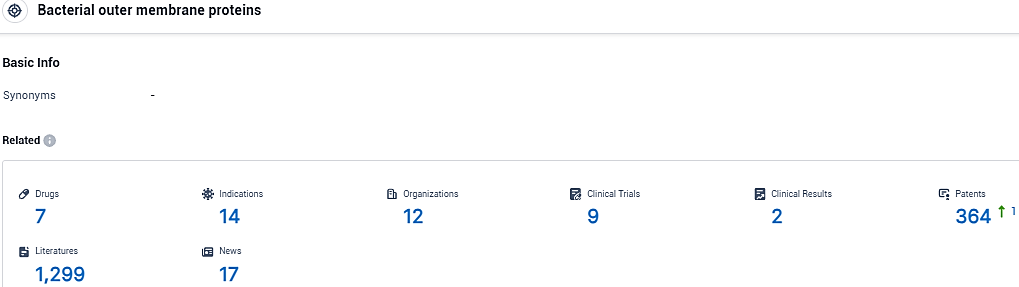
According to the data provided by the Synapse Database, As of November 26, 2023, there are 7 investigational drugs for the Bacterial outer membrane proteins target, including 14 indications, 12 R&D institutions involved, with related clinical trials reaching 9, and as many as 364 patents.
OMN6 is a novel, first-in-class antimicrobial peptide based on insect host defense peptides. Its mechanism of action is based on disruption of bacterial cell membranes and is therefore effective regardless of bacterial genotype or resistance phenotype, and unlike conventional bacteriostatic antibiotics, it is fast acting and bactericidal. OMN6, the Company's lead compound, is intended for the treatment of life-threatening infections caused by Gram-negative bacteria such as Acinetobacter baumannii.