Revumenib: A Quick Look at Its R&D Progress and Clinical Results from the 2023 ASH
The latest clinical findings of Revumenib will be unveiled at the 2023 ASH Congress, demonstrating its potential effect and setting the stage for subsequent investigations.
Revumenib's R&D Progress
Revumenib is a small molecule drug that targets MLL1 and menin. It is being developed by Vitae Pharmaceuticals LLC. The drug has shown potential in treating various therapeutic areas including neoplasms, digestive system disorders, immune system diseases, and hemic and lymphatic diseases.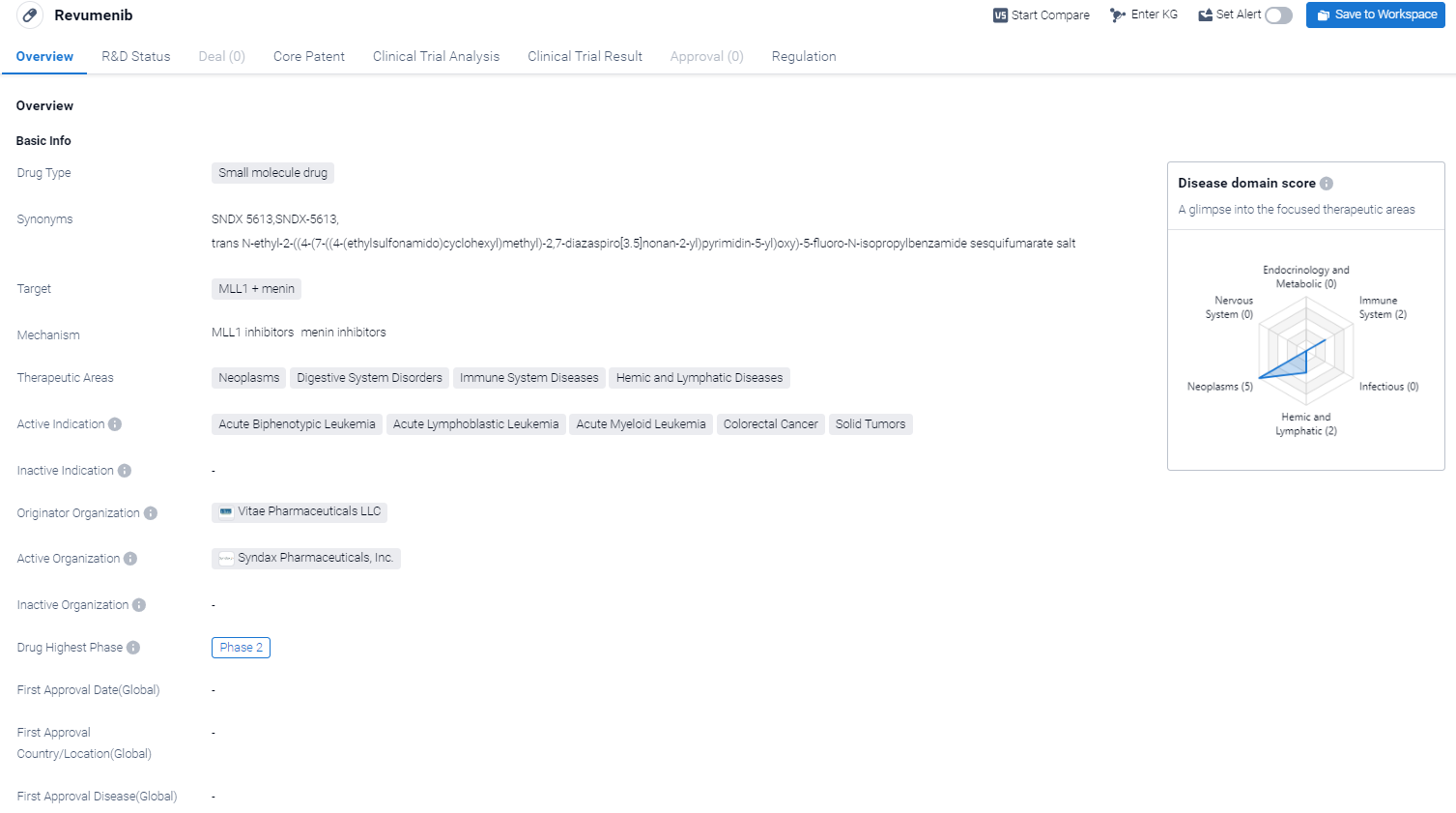
According to the Patsnap Synapse, Revumenib is currently in Phase 2 of clinical trials. And the clinical trial area for Revumenib is primarily in the United States, France and Germany. The key indication is Acute Myeloid Leukemia. 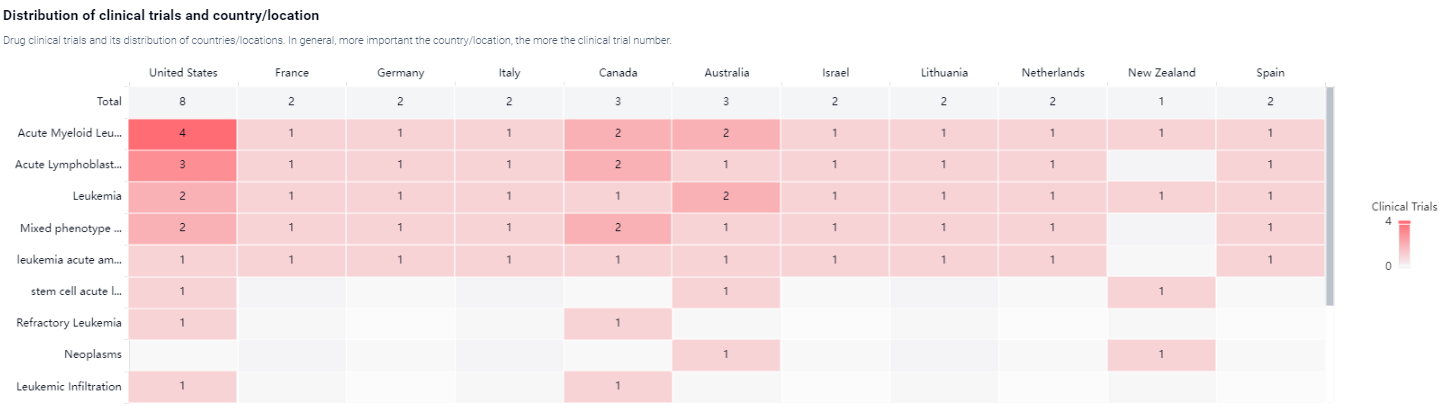
Detailed Clinical Result of Revumenib
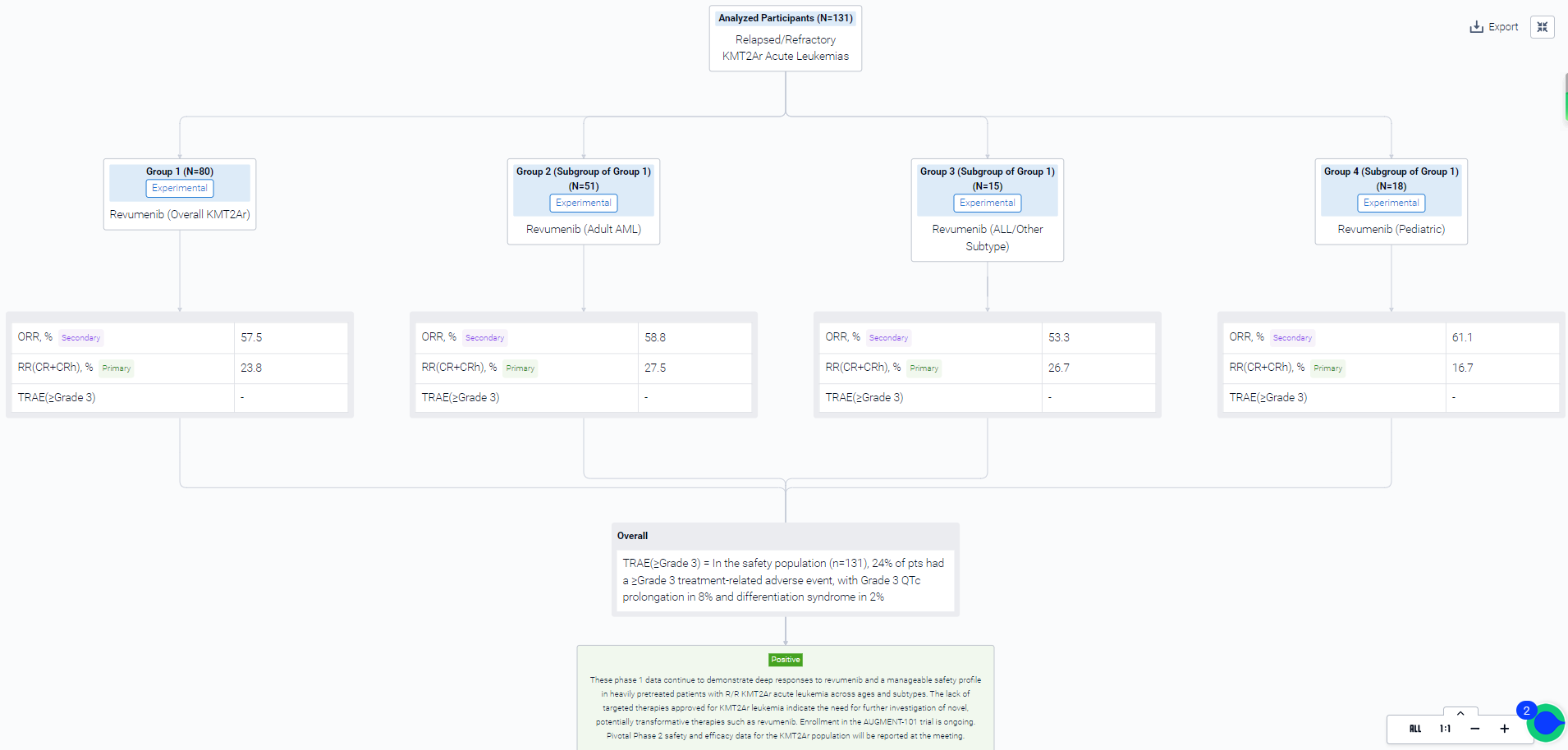
This sequential assignment, open Labeled study (NCT04065399) was aimed to evaluate the efficacy and safety of Revumenib monotherapy in patients with relapsed/refractory KMT2Ar acute leukemias.
In this study, in phase 1, pts aged ≥30 days with R/R acute leukemias were assigned to 1 of 6 dose-escalation arms designed to identify a recommended phase 2 dose (RP2D) for concomitant administration of weak, moderate, or strong cytochrome P450 3A4 inhibitor (CYP3A4i) azole antifungal or no CYP3A4i. Pts received revumenib every 12 hours (q12h) or 3 times a day at a flat dose (≥40 kg) or a body surface area–based dose (<40 kg). Safety was assessed in all pts who received ≥1 dose of revumenib; responses are reported for all treated pts with KMT2Ar. In pts who underwent hematopoietic stem cell transplant (HSCT), responses reported are those achieved before HSCT. The Phase 2 study was initiated following identification of an RP2D of 163 mg (95 mg/m2 if <40 kg) q12h with a strong CYP3A4i in 28-day cycles until unacceptable toxicity, lack of at least morphological leukemia-free state (MLFS) by end of cycle 4, or progressive disease without clinical benefit as defined by the investigator. The phase 2 primary objectives are evaluation of safety and tolerability of revumenib and assessment of the complete remission (CR) + CR with partial hematologic recovery (CRh) rate. The safety and efficacy of revumenib at an RP2D is being explored in 3 expansion cohorts: Cohort 2A, KMT2Ar acute lymphoblastic leukemia (ALL)/mixed phenotype acute leukemia; Cohort 2B, KMT2Ar acute myeloid leukemia (AML); and Cohort 2C, NPM1m AML. A preplanned pooled analysis of adult and pediatric pts with KMT2Ar acute leukemia will be conducted when a subset of Cohorts 2A+2B pts have completed a minimum of 4 cycles or have discontinued therapy.
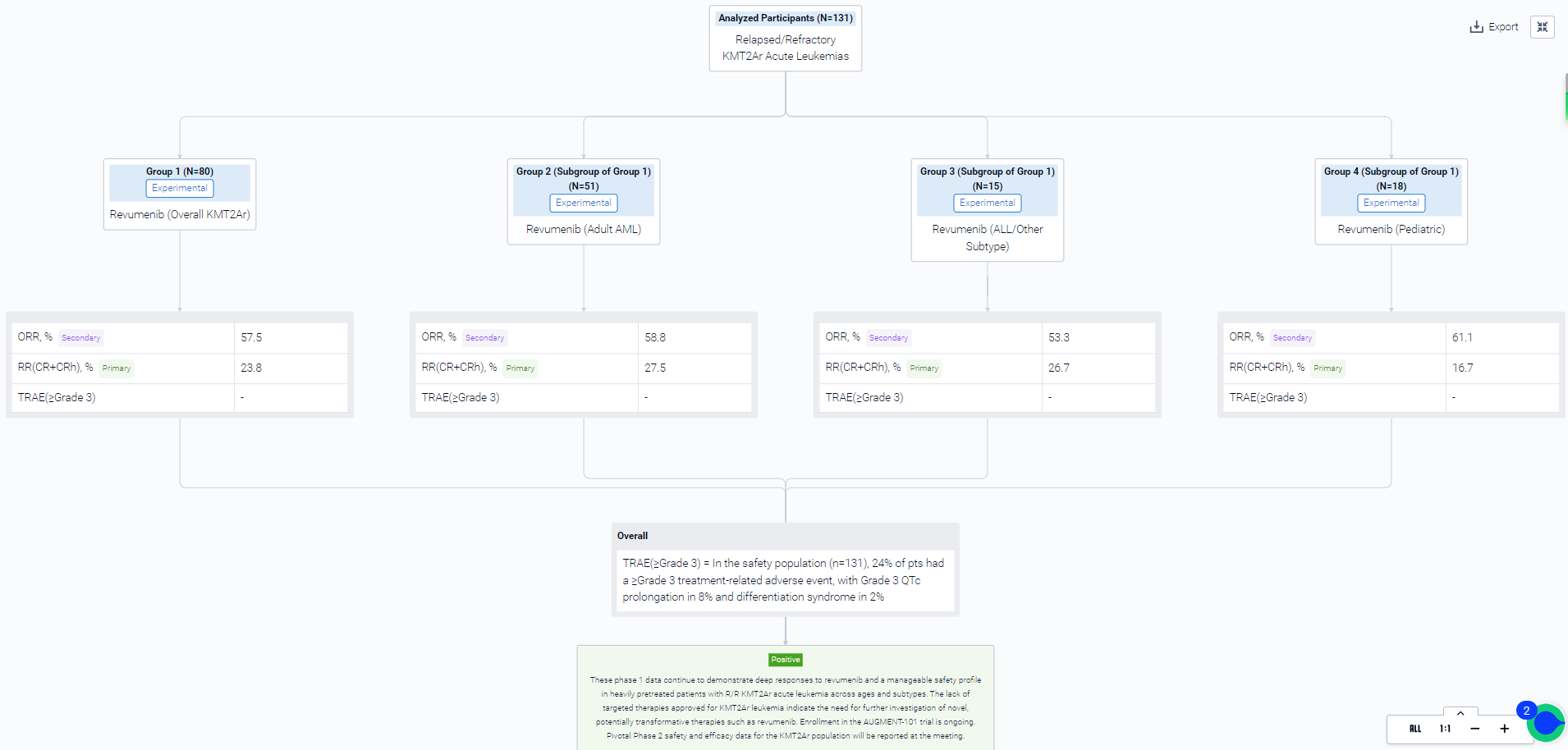
The result showed that phase 1: as of June 19, 2023, 80 pts with R/R KMT2Ar acute leukemia were treated with revumenib in the 6 dose-escalation arms. In adults with AML (n=51), the largest KMT2Ar subgroup, CR+CRh was 28% and overall response rate (ORR) was 59%. Responses were consistent in ALL/other leukemia subtype (n=15), with 27% CR+CRh and 53% ORR. ORR (61%) was similar in pediatric pts (<18 years, n=18), with 22% proceeding to HSCT. Although similar percentages of adult and children proceeded to transplant, consistent with common clinical practice in pediatrics, 75% of children transplanted went on to HSCT prior to achieving a CR/CRh (2 MLFS, 1 CRp) versus 17% of adults. In the safety population (n=131), 24% of pts had a ≥Grade 3 treatment-related adverse event (TRAE), with Grade 3 QTc prolongation in 8% and differentiation syndrome in 2%. QTc prolongation remained as the only dose-limiting toxicity; no patients experienced ventricular arrhythmias. In addition, based on updated phase 1 pharmacokinetics, clinical activity, and safety data, an RP2D of 276 mg q12h without a strong CYP3A4i was established.
Phase 2: In a preliminary analysis of Cohorts 2A and 2B (June 19, 2023), 88 pts with KMT2Ar acute leukemia were enrolled and treated with revumenib at an RP2D (Table 2). Ages ranged from 1.3 to 75 years (22 aged <18 y). Leukemia subtypes included ALL (15%), AML (81%), and other (3%). Pts were heavily pretreated, having received a median of 3 prior lines of therapy (range 1-12); 38% received ≥4 prior lines and 48% had prior HSCT.
It can be concluded that these phase 1 data continue to demonstrate deep responses to revumenib and a manageable safety profile in heavily pretreated patients with R/R KMT2Ar acute leukemia across ages and subtypes. The lack of targeted therapies approved for KMT2Ar leukemia indicate the need for further investigation of novel, potentially transformative therapies such as revumenib. Enrollment in the AUGMENT-101 trial is ongoing. Pivotal Phase 2 safety and efficacy data for the KMT2Ar population will be reported at the meeting.
How to Easily View the Clinical Results Using Synapse Database?
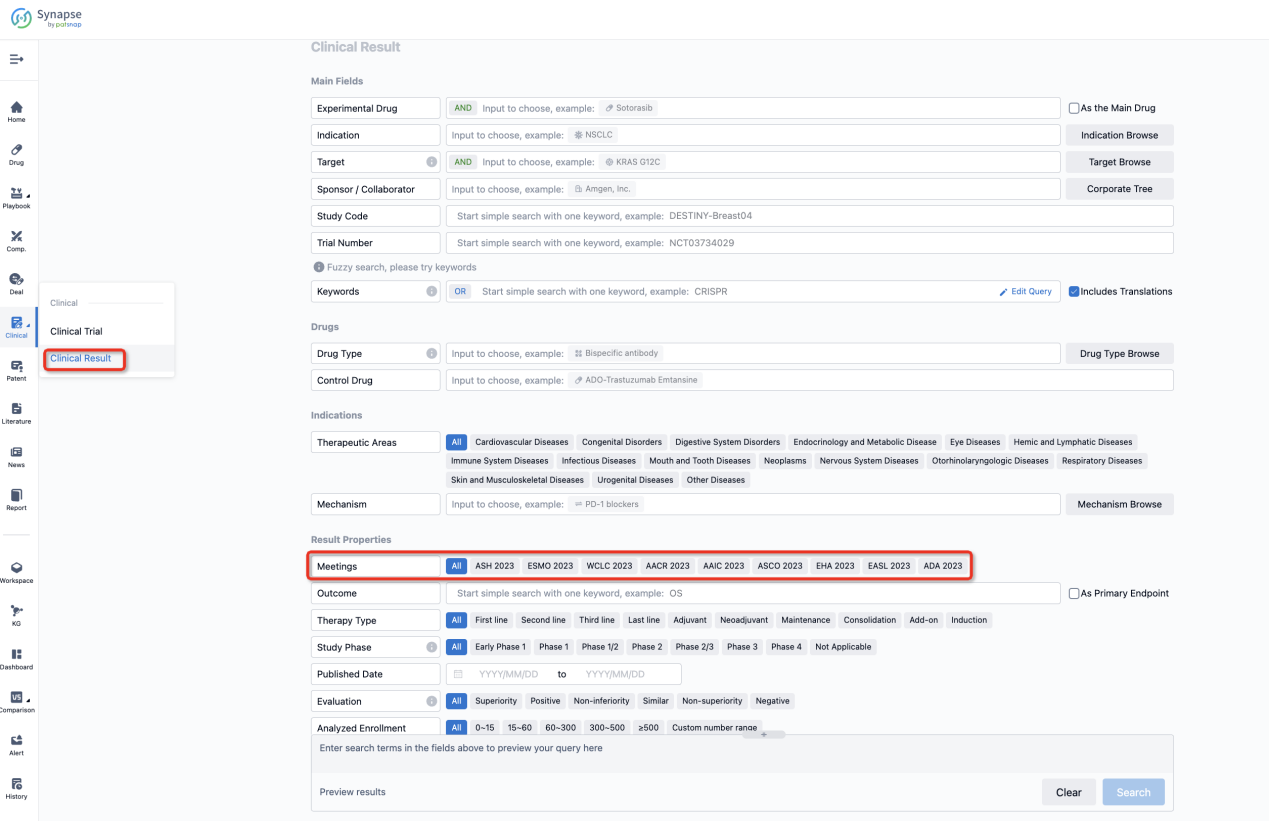
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
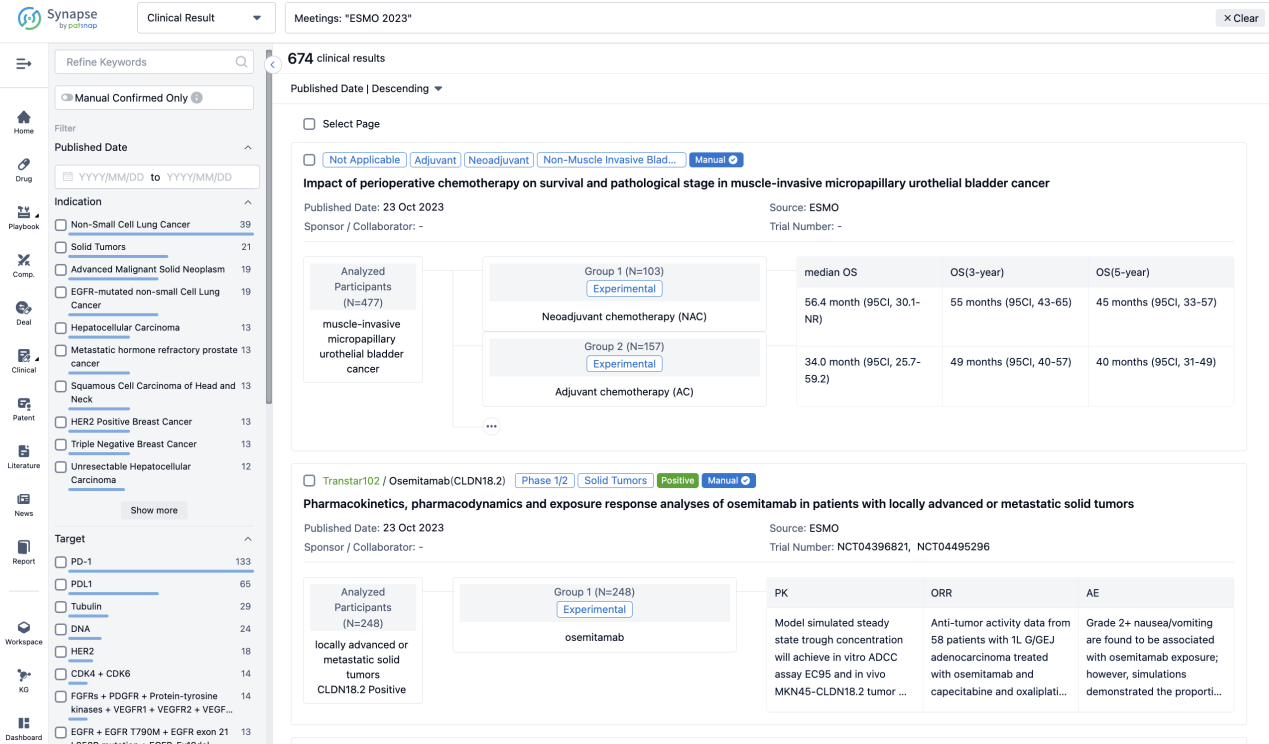
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
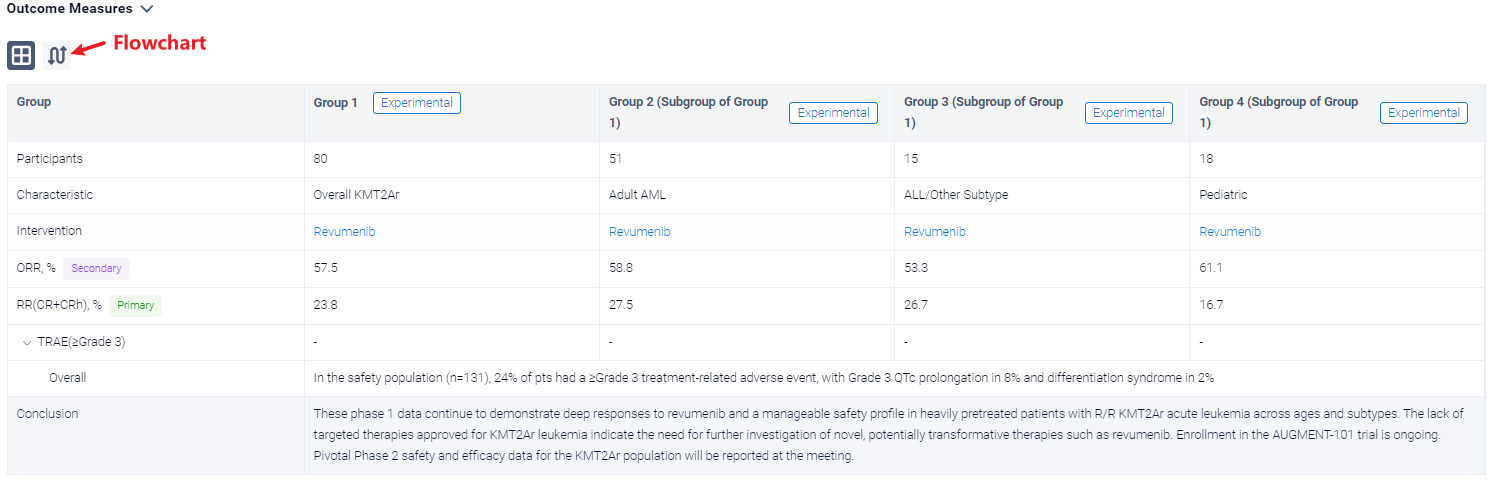
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
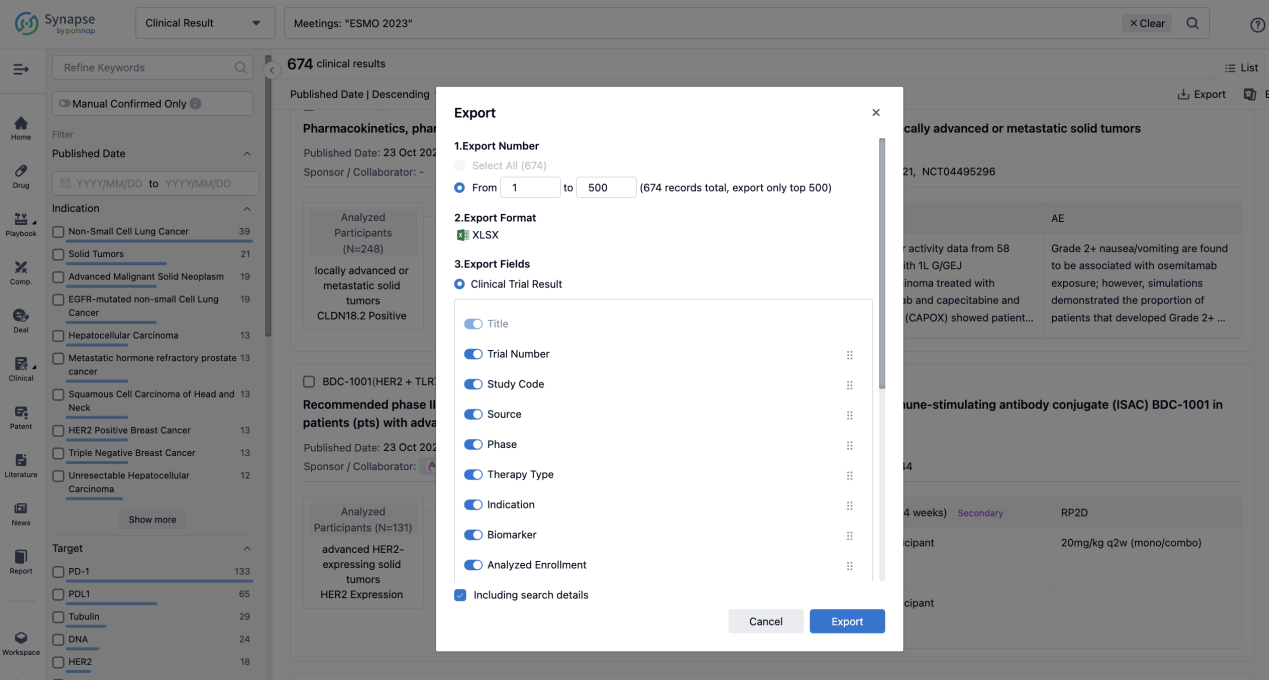
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!