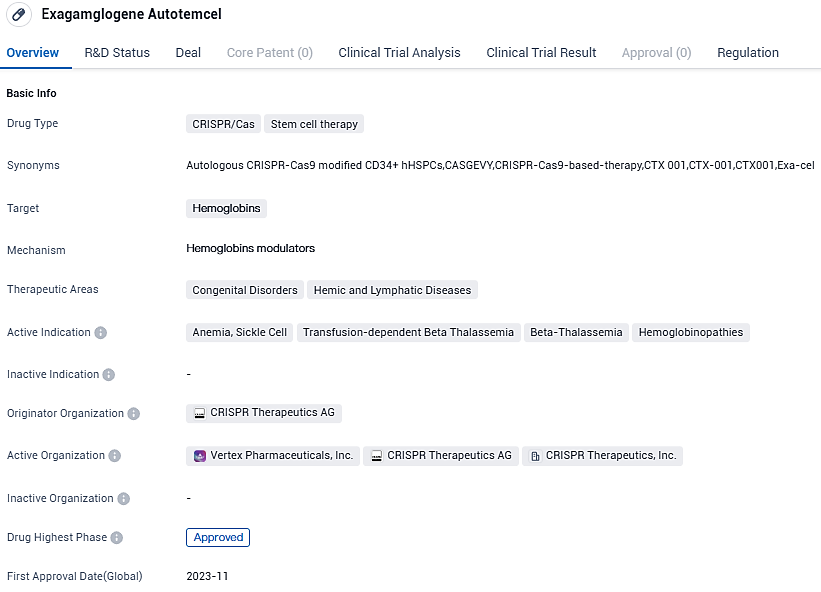
UK's MHRA approves CRISPR and Vertex's gene-editing treatment, CASGEVY, for Sickle Cell and Beta Thalassemia using CRISPR/Cas9
It has been announced by Vertex Pharmaceuticals Incorporated in alliance with CRISPR Therapeutics that CASGEVY™ (exagamglogene autotemcel [exa-cel]), a therapeutic intervention genetically edited using CRISPR/Cas9 technology, has successfully obtained a provisional go-ahead for marketing from the UK's Medicines and Healthcare products Regulatory Agency. This authorization brings new hope for treatment options for patients suffering from both sickle cell ailment and transfusion-reliant beta thalassemia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
CASGEVY has received authorization to treat qualified patients aged 12 and up with SCD who are experiencing regular vas-occlusive crises or TDT, in cases where a related hematopoietic stem cell donor, with human leukocyte antigen compatibility, is not readily available. The estimation is that about 2,000 patients could benefit from CASGEVY across the UK.
"I trust this is only the beginning of many implementations of this Nobel Prize-recognized technology that can assist patients with severe diseases," commented Samarth Kulkarni, Ph.D., CRISPR Therapeutics' CEO and Chairman.
In two international clinical trials targeting SCD and TDT patients with CASGEVY, the trials reached their primary goal of patients remaining free from extreme VOCs or being transfusion independant over a span of 12 straight months. If fulfilled, such benefits are possibly thought to be lifelong. The characterization of safety for 97 patients with SCD and TDT that have been treated with CASGEVY thus far in these ongoing trials generally aligns with the myeloablative conditioning with busufan and hematopoietic stem cell transplant.
Professor Josu de la Fuente, Principal Investigator for the CLIMB-111 and CLIMB-121 studies, Professor of Practice at Imperial College London, and Consultant Haematologist for Imperial College Healthcare NHS Trust, said, "This approval provides an alternative for deserving patients awaiting novel treatments, and I eagerly anticipate patients receiving this treatment as swiftly as possible."
In the UK, the MHRA issued an Innovation Passport to exa-cel under the Innovative Licensing and Access Pathway, and Vertex is collaborating intensely with national health authorities to guarantee speedy access for deserving patients.
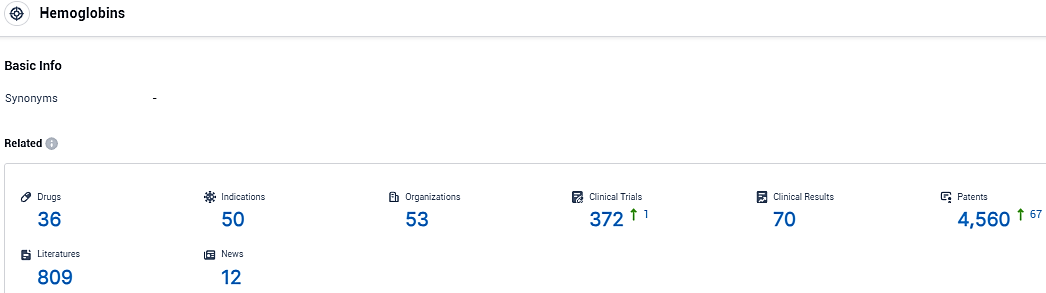
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 22, 2023, there are 36 investigational drugs for the Hemoglobins target, including 50 indications, 53 R&D institutions involved, with related clinical trials reaching 372, and as many as 4560 patents.
CASGEVY™ is also under review by the European Medicines Agency, the Saudi Food and Drug Authority, and the U.S. Food and Drug Administration (FDA). The FDA has granted Priority Review for SCD and Standard Review for TDT and assigned Prescription Drug User Fee Act (PDUFA) target action dates of December 8, 2023, and March 30, 2024, respectively.