Exploring RBN-2397's R&D successes and its clinical results at the 2023 AACR
On 14 Apr 2023, the first-in-class first-in-human phase 1 trial and translational study of the mono (ADP-ribose) polymerase-7 (PARP7) inhibitor RBN-2397 was reported at the AACR Congress.
RBN-2397's R&D Progress
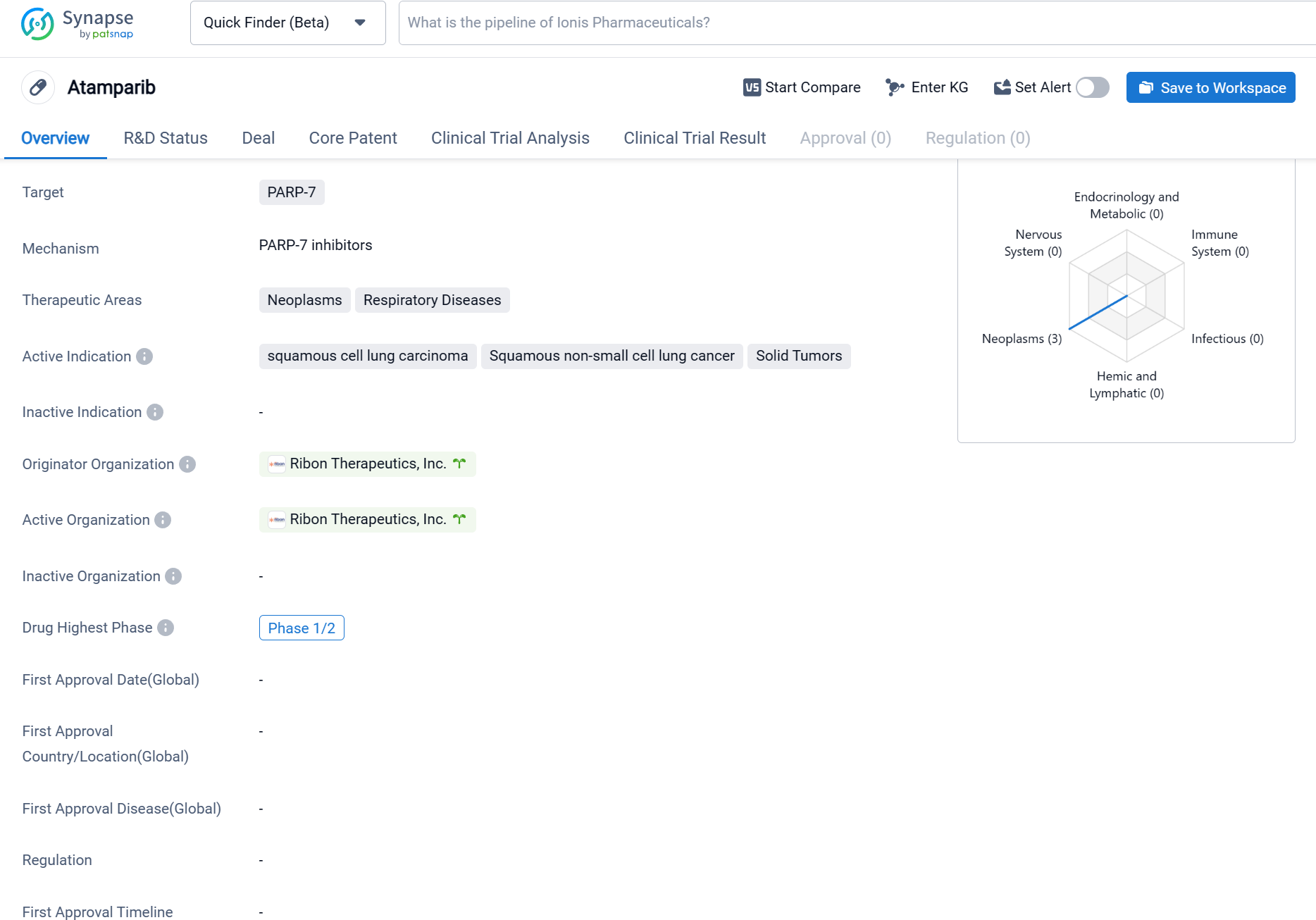
RBN-2397 is a small molecule drug that falls under the therapeutic area of neoplasms and respiratory diseases. It specifically targets PARP-7, a protein involved in DNA repair processes. The drug is being developed to treat various types of cancers, including squamous cell lung carcinoma, squamous non-small cell lung cancer, and solid tumors.
According to the Patsnap Synapse, RBN-2397 has reached the highest phase of clinical trials, which is Phase 1/2. And the clinical trial areas for RBN-2397 are primarily in the United States, United Kingdom, and Israel. The key indication is Small Cell Lung Cancer. 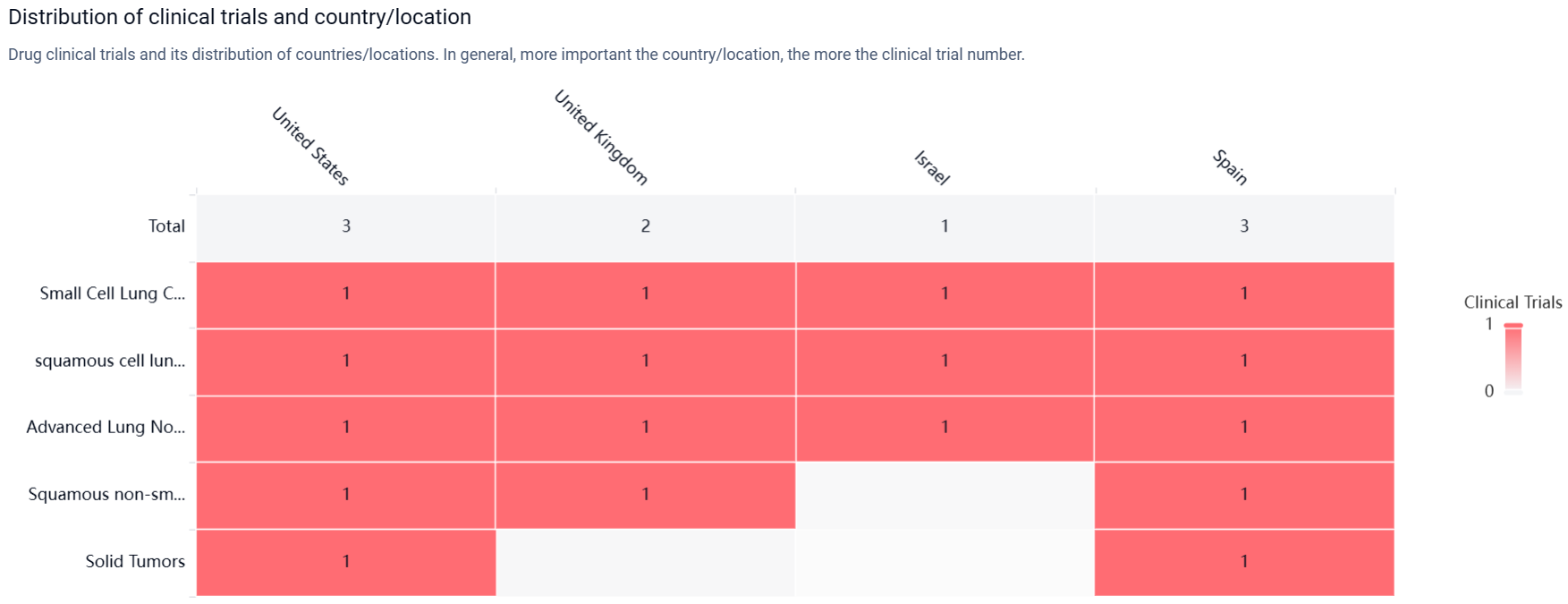
Detailed Clinical Result of RBN-2397
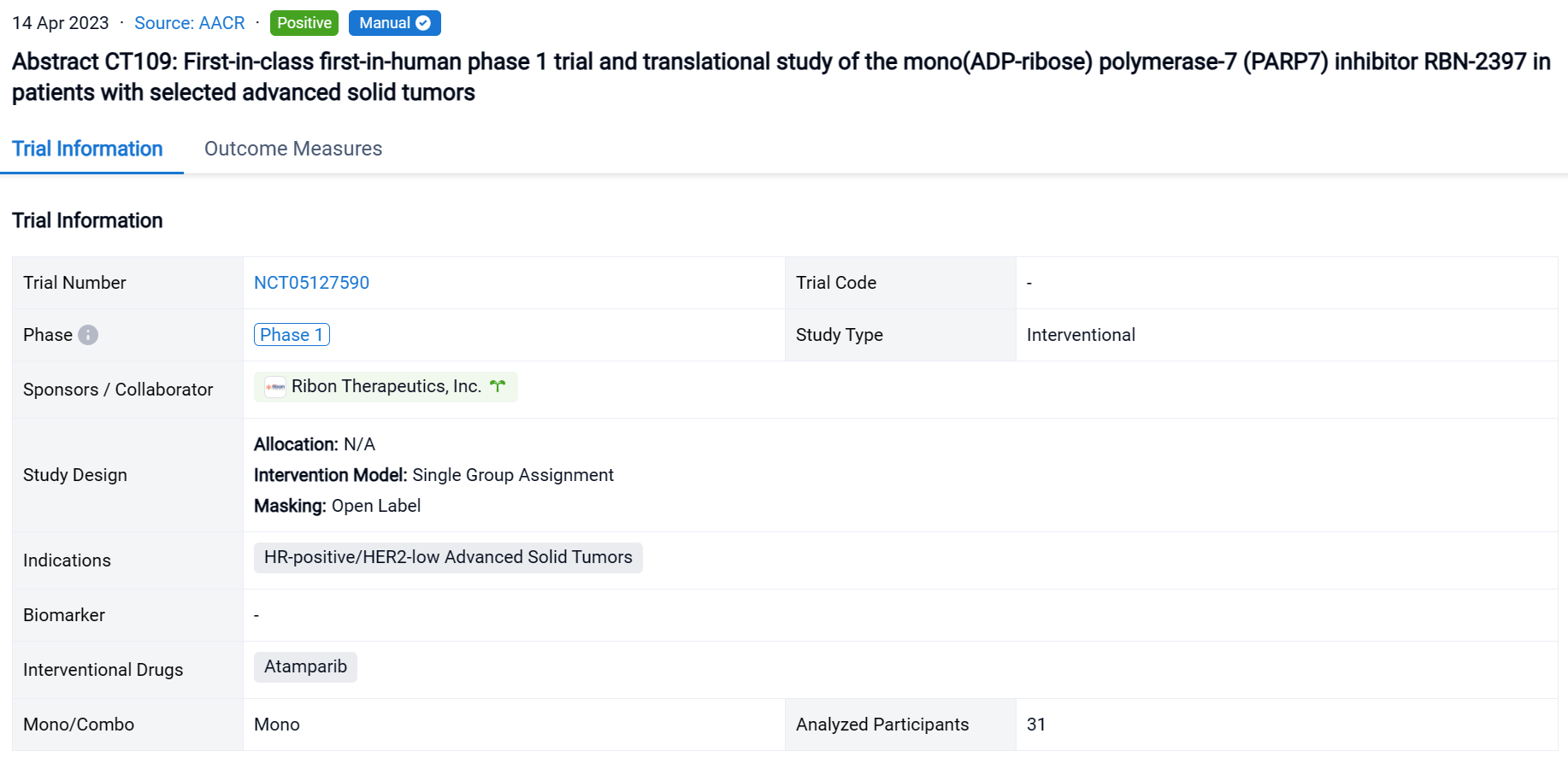
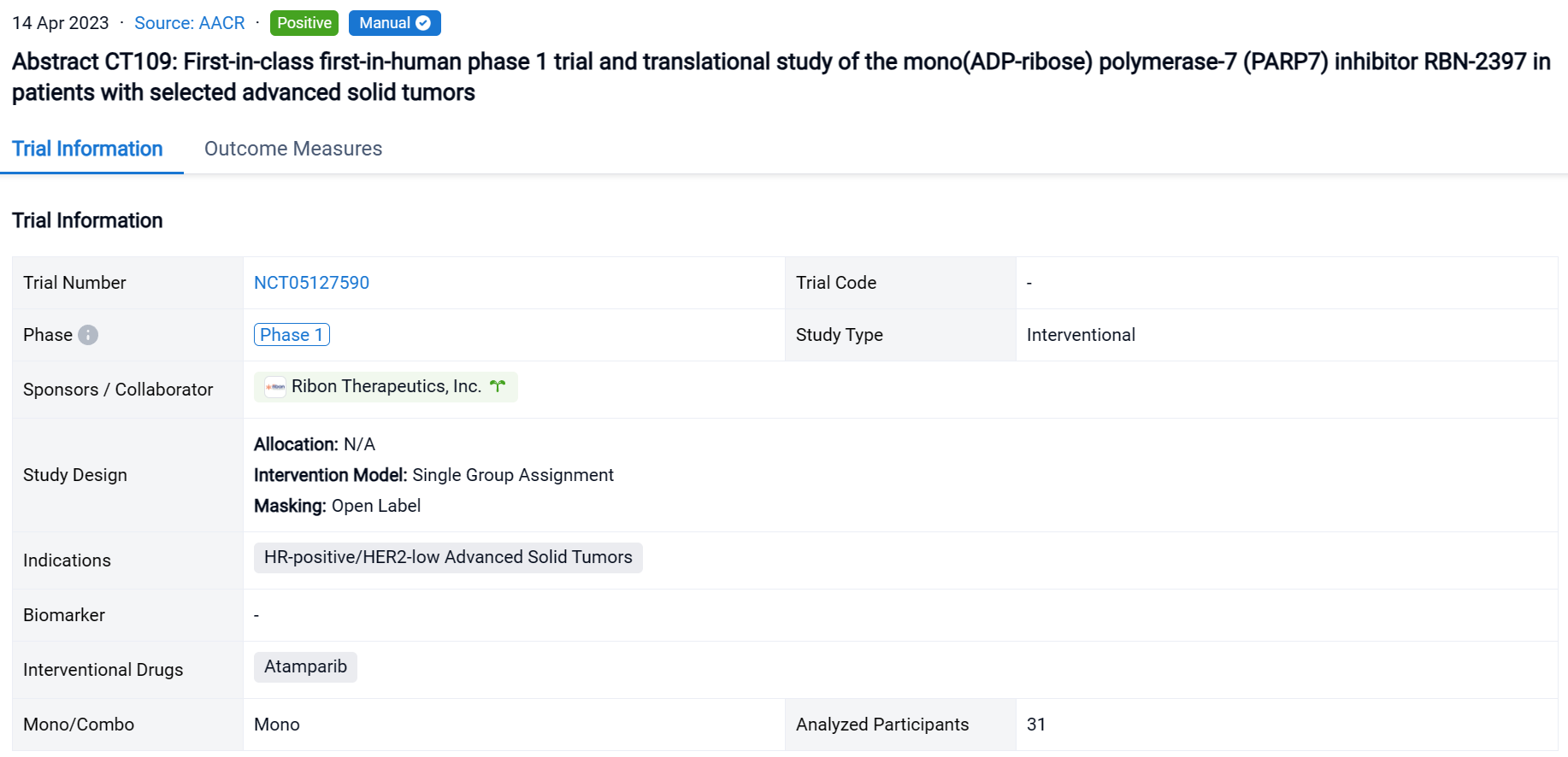
The single group assignment, open-labeled clinical trial (NCT05127590) was conducted in patients with selected advanced solid tumors.
In this study, pts with solid tumors were treated with RBN-2397 at the RP2D of 200 mg BID in 3 expansion cohorts: squamous cell carcinoma of the lung (SCCL), head and neck squamous cell carcinoma (HNSCC), and hormone receptor-positive breast cancer (HR+ BC). Objectives of the expansion phase included safety, pharmacokinetics, pharmacodynamics, and antitumor activity.
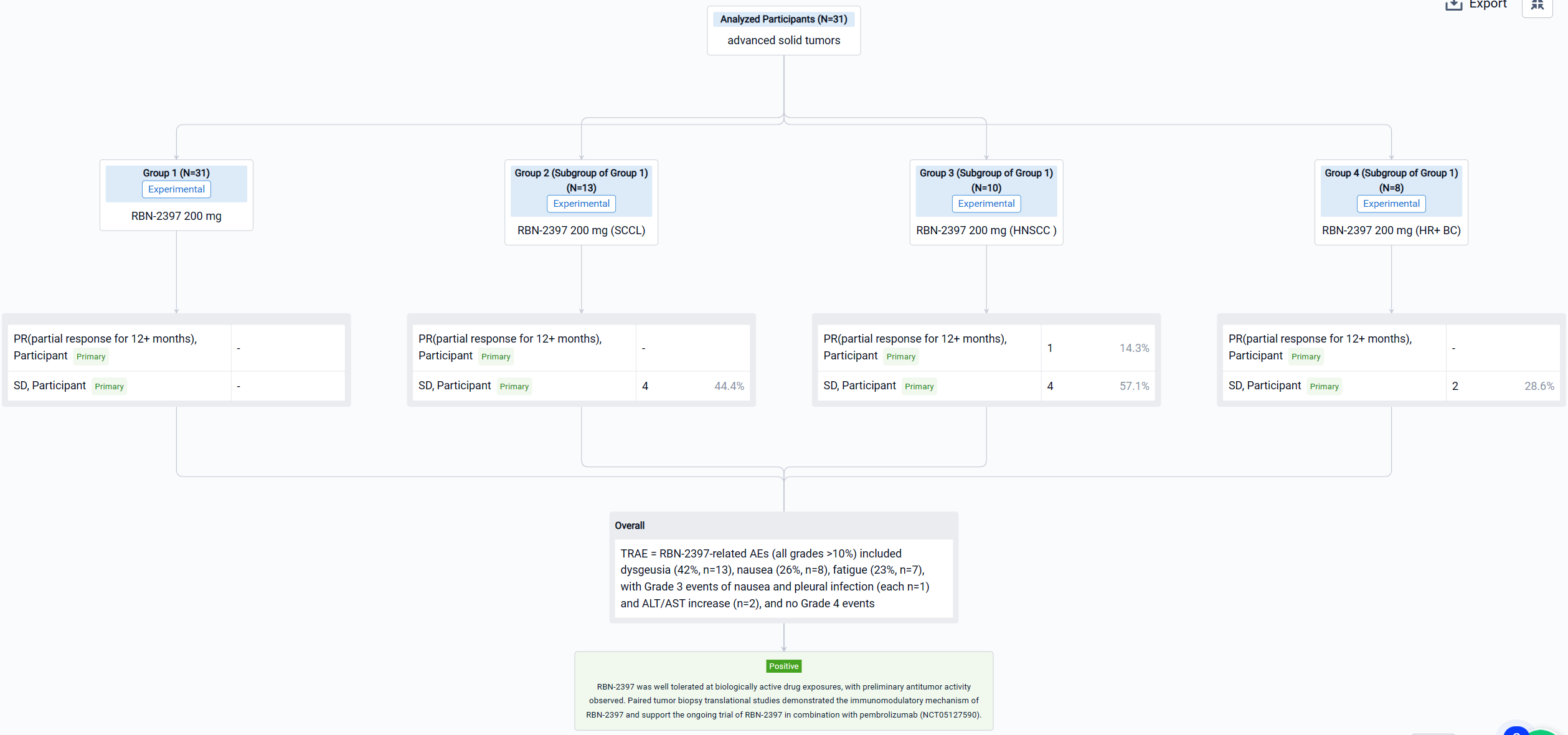
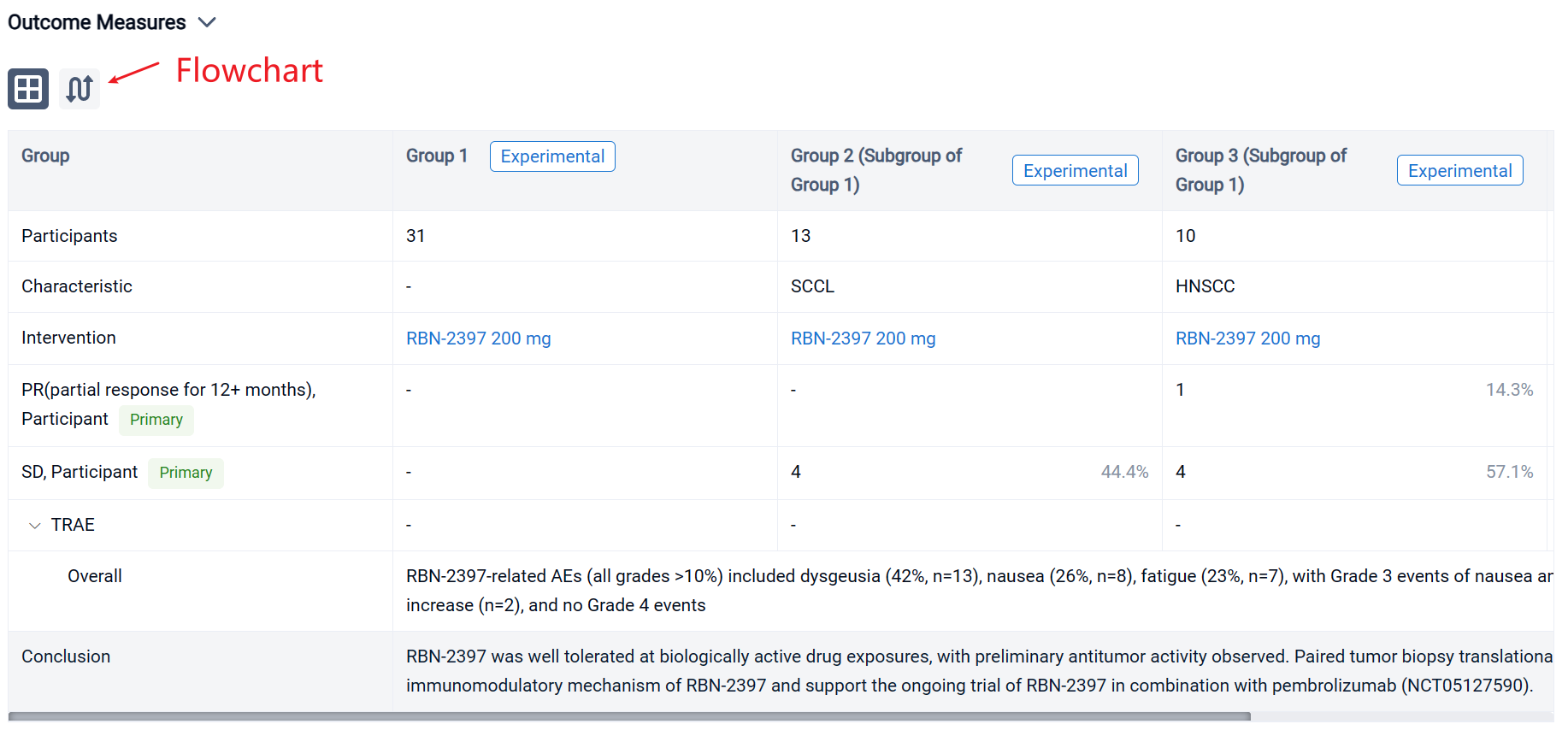
The result showed that as of 2 July 2022, 31 pts have been treated: SCCL (n=13), HNSCC (n=10), and HR+ BC (n=8). RBN-2397-related AEs (all grades >10%) included dysgeusia (42%, n=13), nausea (26%, n=8), fatigue (23%, n=7), with Grade 3 events of nausea and pleural infection (each n=1) and ALT/AST increase (n=2), and no Grade 4 events. No significant chronic toxicities were observed. The disease control rate in response-evaluable pts was 44% in SCCL (stable disease [SD] in 4/9 pts), 71% in HNSCC (RECIST partial response [PR] for 12+ months in 1/7; SD in 4/7), and 29% in HR+ BC (SD in 2/7). Analyses of paired tumor biopsies confirmed induction of adaptive immunity with ≥2-fold increases in CD8+ T cells and/or granzyme B expression in 10 (63%) of 16 pts across tumor types. Increases in immune checkpoint expression (PD-1 and LAG3 on T cells; PD-L1 on tumor cells) from 30% to 150% were observed in 3 (60%) of 5 pt tumor samples evaluated using MIBI-SCOPE, indicating the potential to prime tumors for immune checkpoint inhibitor therapy.
It can be concluded that RBN-2397 was well tolerated at biologically active drug exposures, with preliminary antitumor activity observed. Paired tumor biopsy translational studies demonstrated the immunomodulatory mechanism of RBN-2397 and support the ongoing trial of RBN-2397 in combination with pembrolizumab (NCT05127590).
How to Easily View the Clinical Results Using Synapse Database?
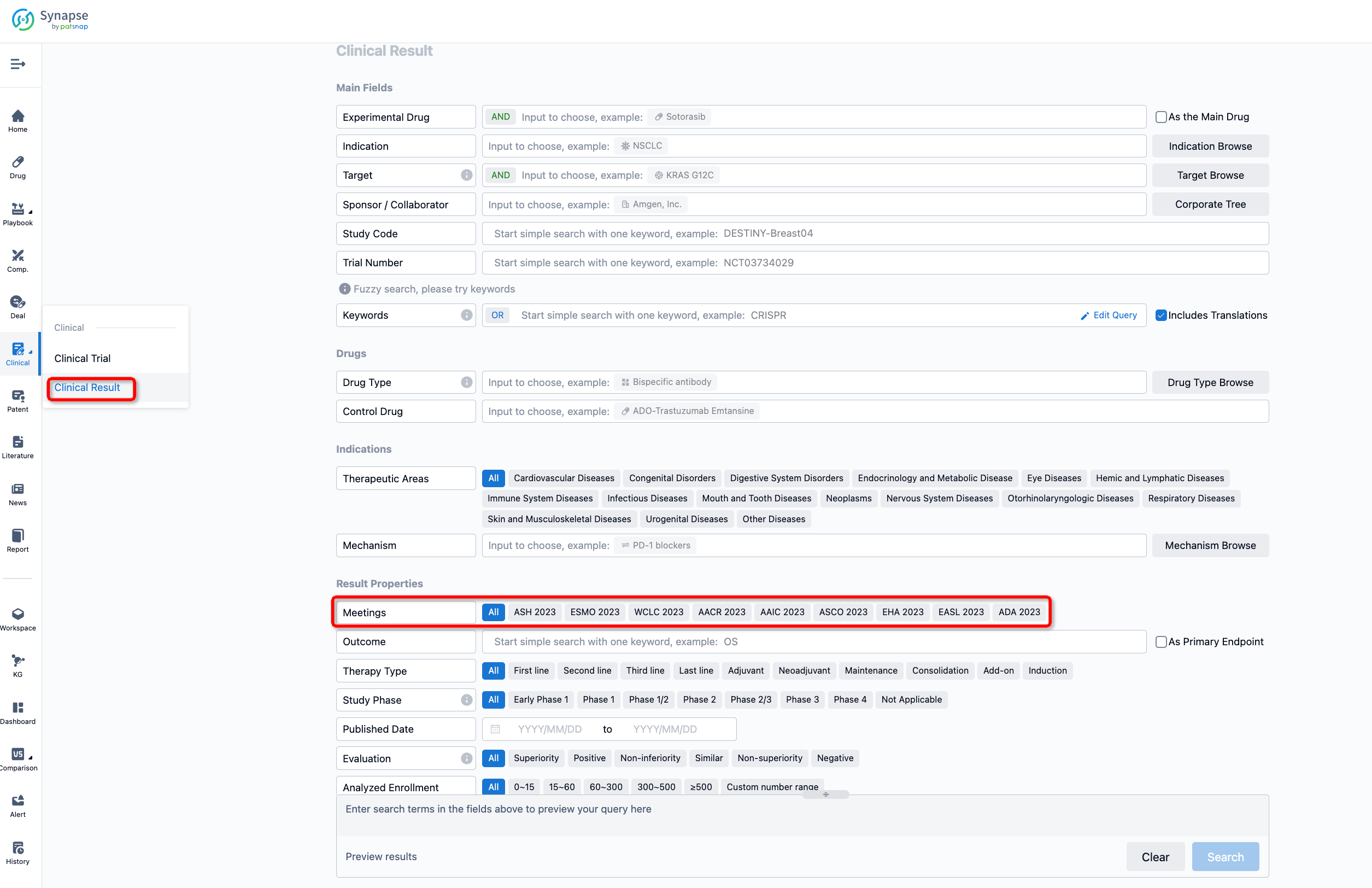
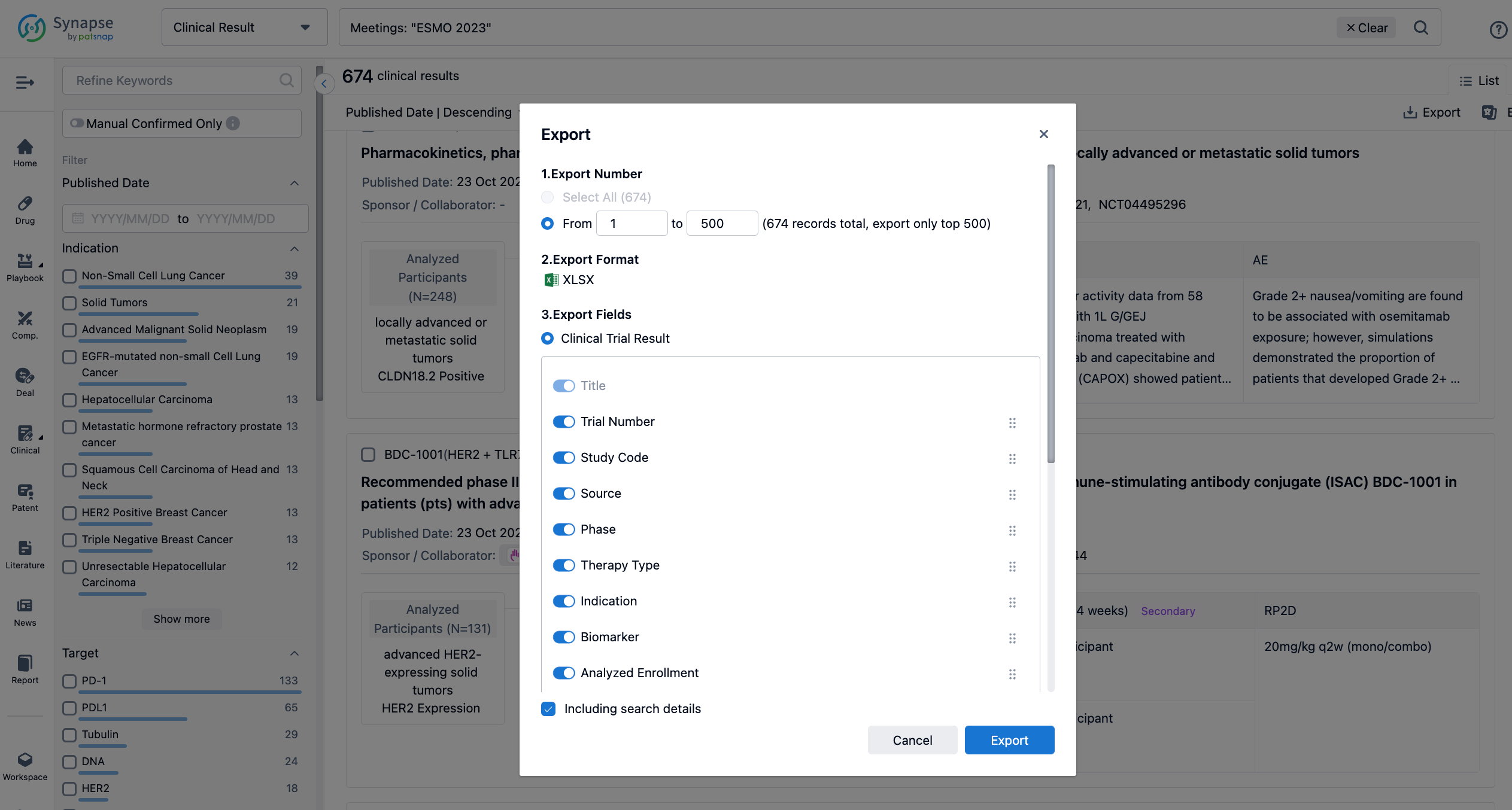
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
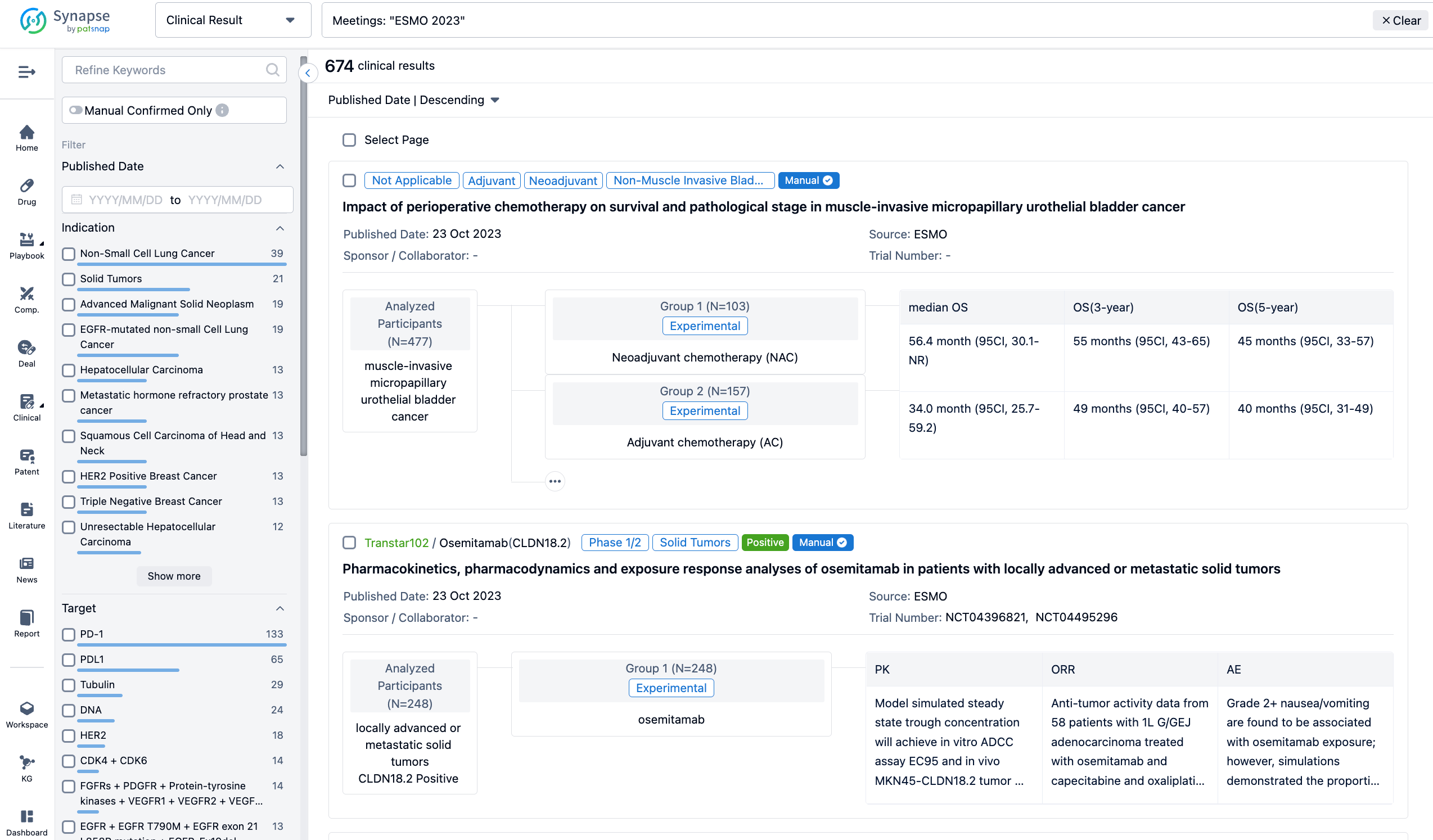
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
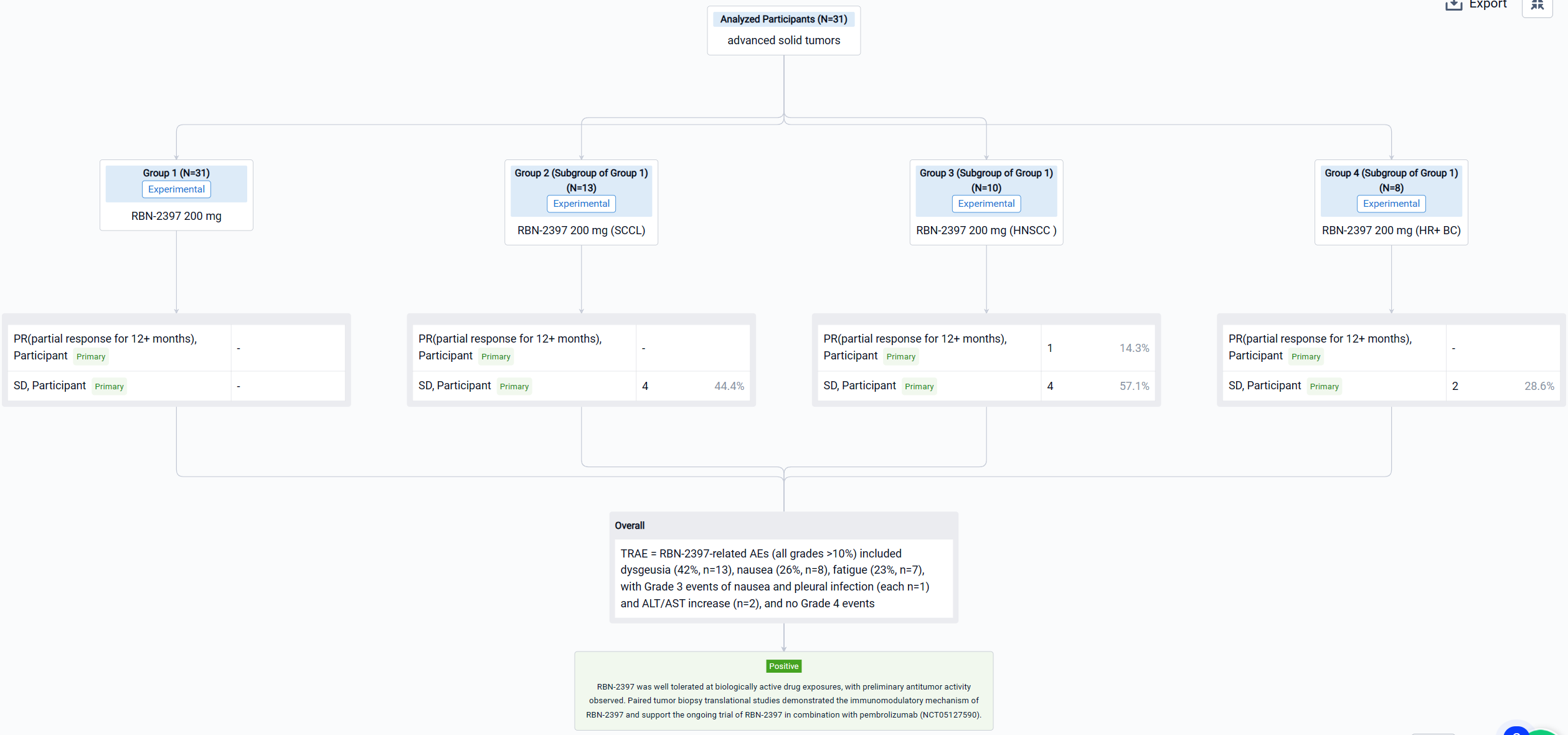
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!