Exploring the Diverse Applications and Patent Landscape of Fentanyl Citrate
Fentanyl Citrate is a small molecule drug that targets the μ opioid receptor and is used to treat a range of therapeutic areas including nervous system diseases, urogenital diseases, and other diseases. Its active indications include breakthrough pain, cancer pain, hyperemesis gravidarum, and anesthesia. The drug was first approved in the United States in 1968 and has since received approval in other countries. The originator organization of Fentanyl Citrate is Rising Pharmaceuticals, Inc. As a small molecule drug, Fentanyl Citrate is designed to act on the μ opioid receptor, which is known to regulate pain. This mechanism of action contributes to its effectiveness in treating pain-related conditions such as breakthrough pain and cancer pain. Additionally, its approval for use in anesthesia indicates its role in managing pain during surgical procedures.
The highest phase of Fentanyl Citrate in both the global and Chinese markets is approved, indicating that it has successfully completed the necessary clinical trials and regulatory processes to be available for medical use. Fentanyl Citrate's multiple approved indications demonstrate its effectiveness in managing various types of pain and its use in anesthesia.
Below, we will use the drug Fentanyl Citrate as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
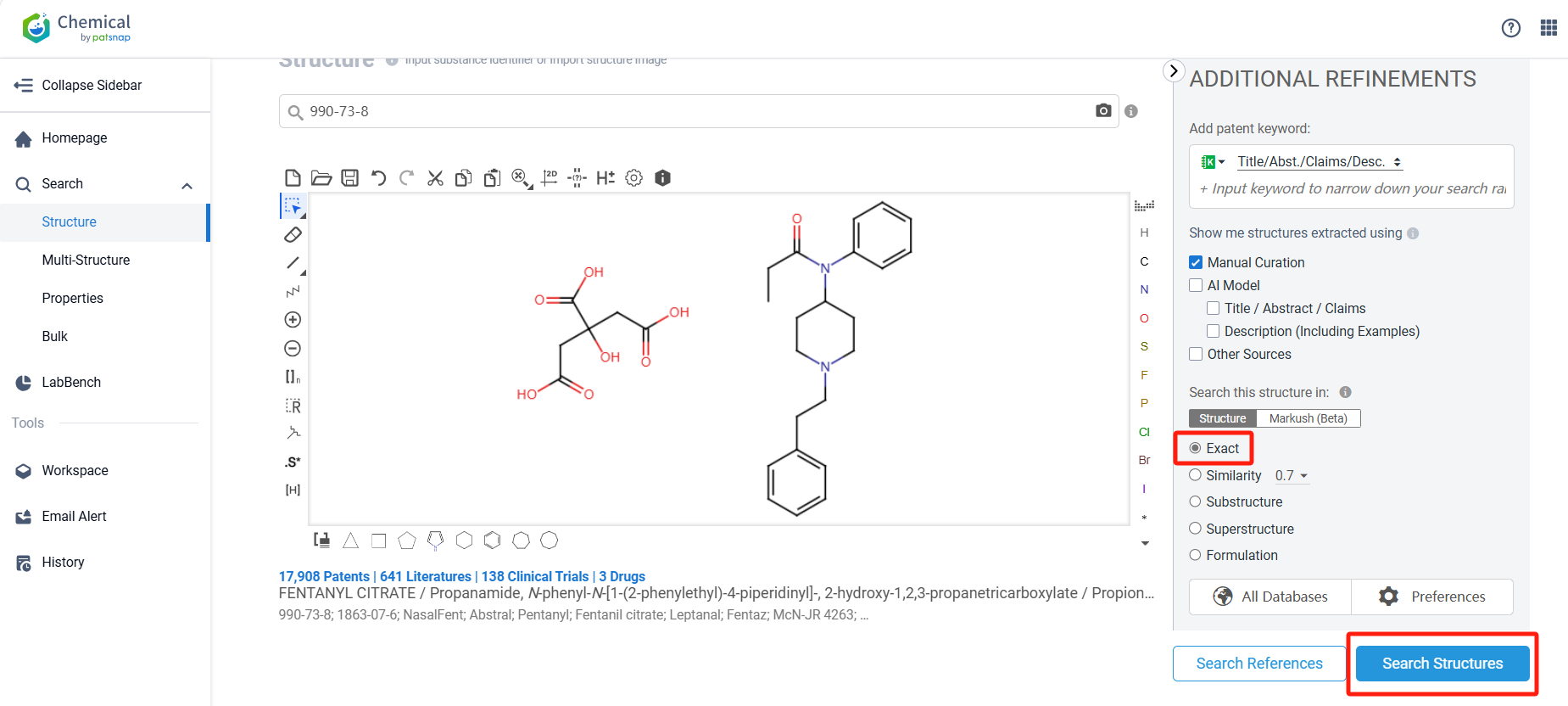
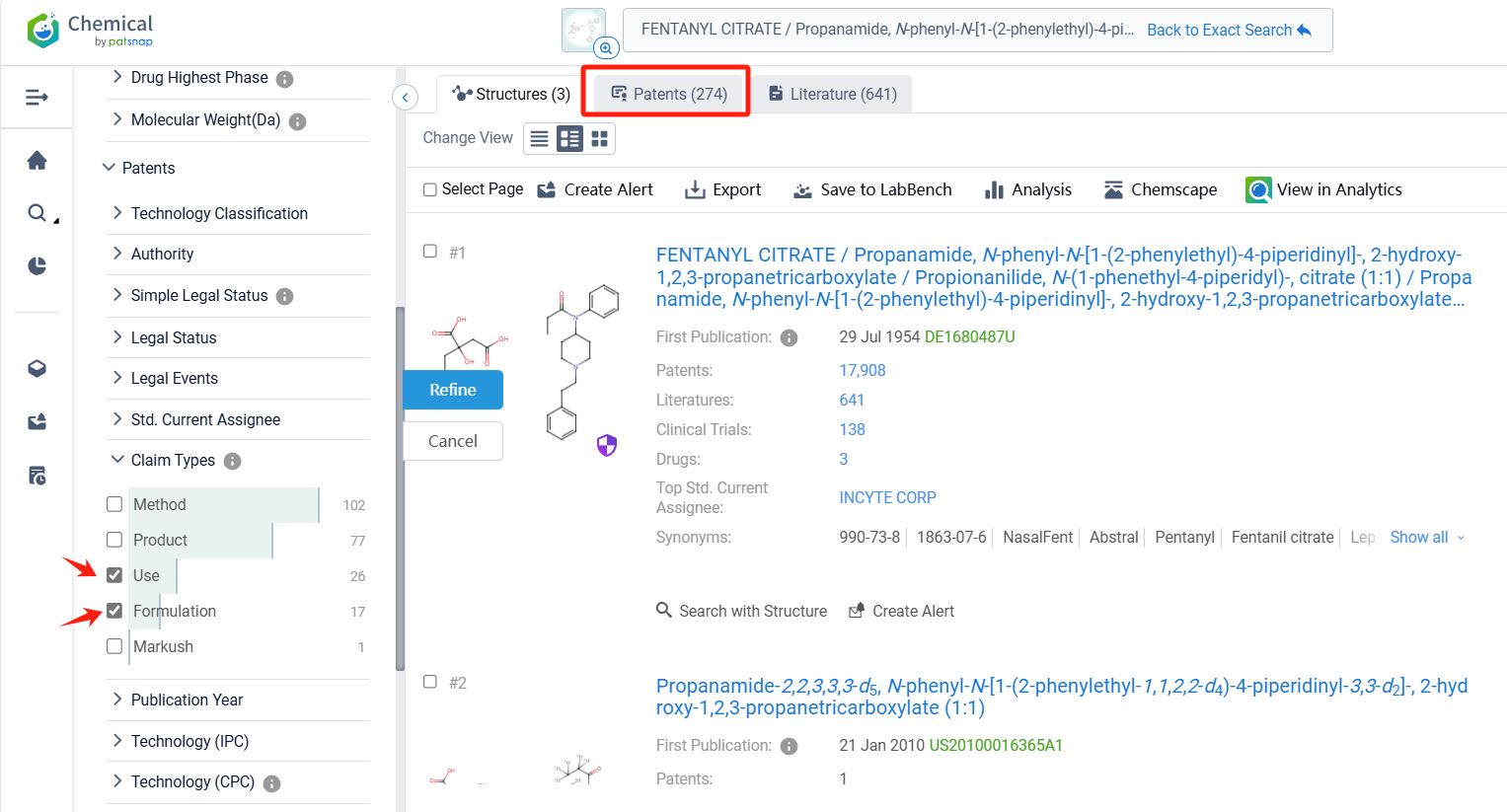
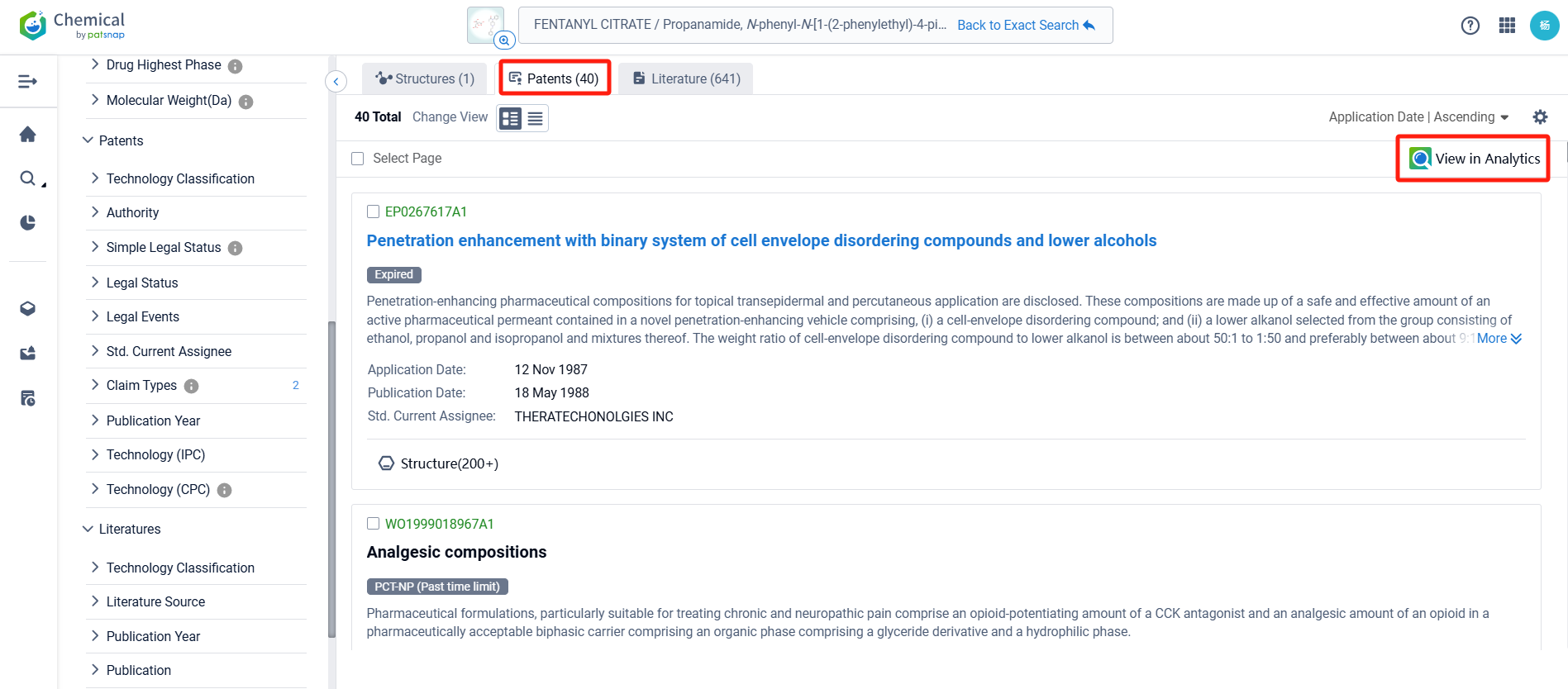
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Fentanyl Citrate (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 274 patents. In the sidebar, select "Formulation" and "Use" under the "Claim Types" to search for patents related to new formulations and new indications. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
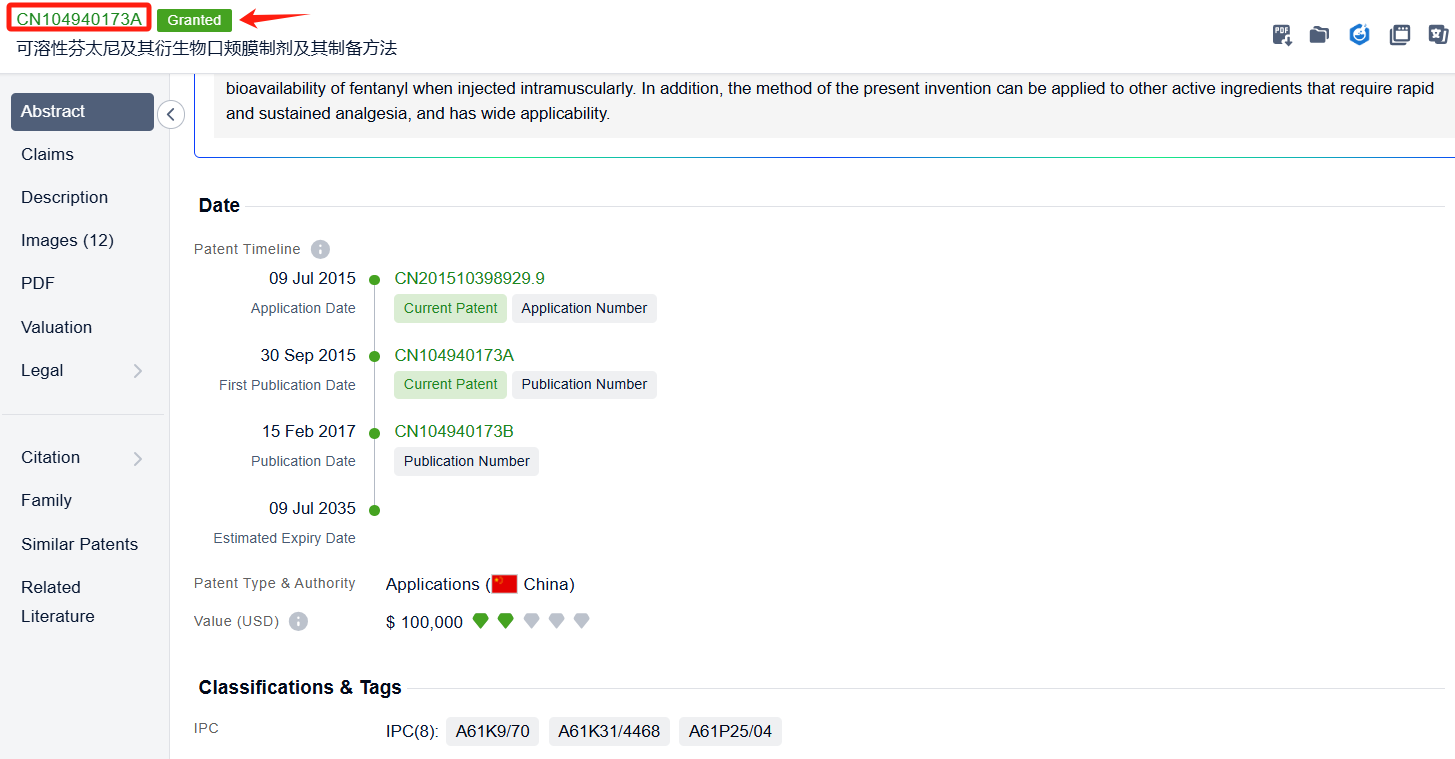
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Fentanyl Citrate. By reviewing the aforementioned patents, we can observe that Transdermal Sedation Solutions LLC's international patent WO2022120263A1(application date 20211206, publication date 20220609) discloses A solid drug matrix formulation comprising: clonidine in an amount ranging from 10-300 pg; midazolam in an amount ranging from 1-40 mg; fentanyl in an amount ranging from 10-300 pg for inducing sedation in a patient before a clinical procedure. Its corresponding patents in the United States has been granted. Additionally, Xiamen LP Pharmaceutical Co., Ltd.'s patent CN104940173B (application date 20150709, Publication Date 20170215) provides a method for preparing a fentanyl instant film, a fentanyl sustained-release film and a fentanyl instant-release double-layer film. The bioavailability reaches more than 80%, which is basically close to the bioavailability of fentanyl injection. degree, which is significantly higher than the 67% bioavailability of fentanyl when injected intramuscularly. 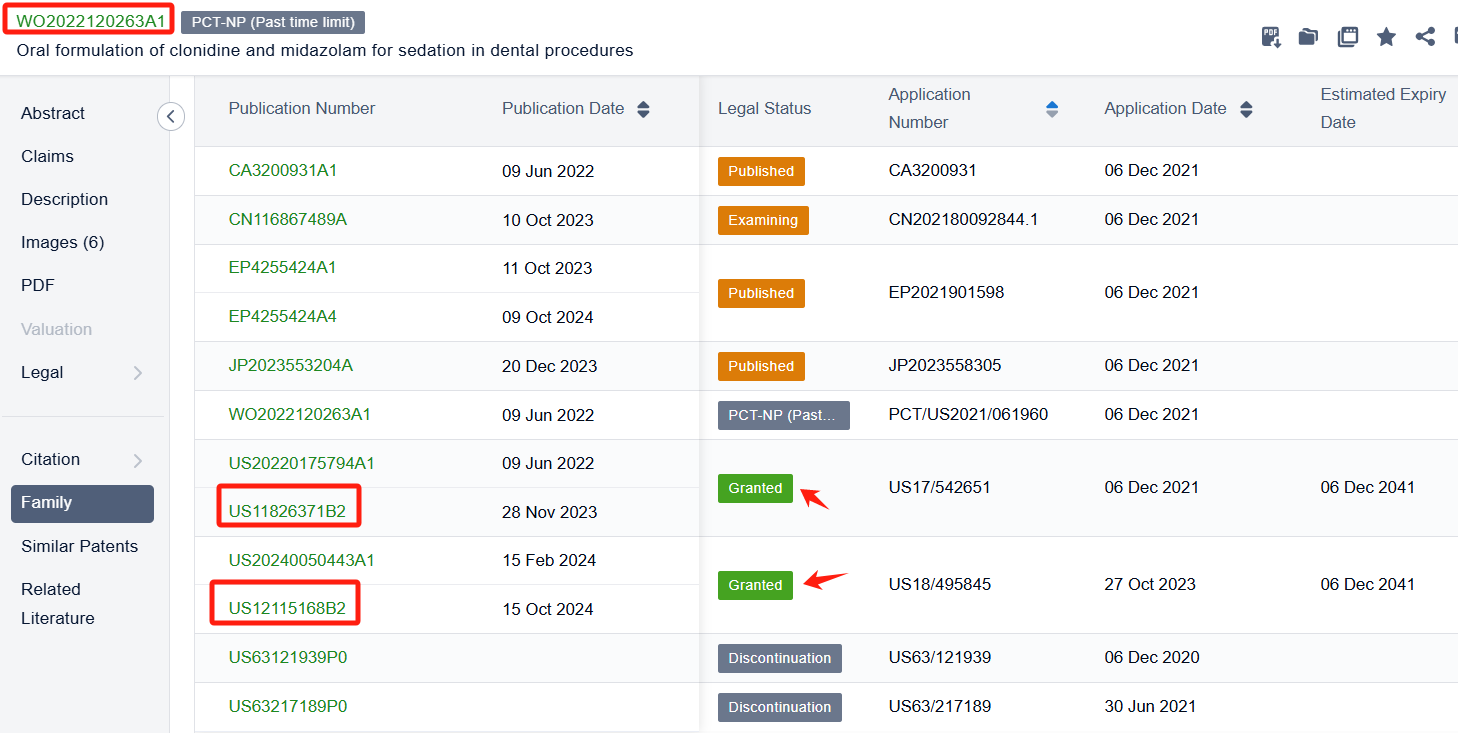
The therapeutic areas in which Fentanyl Citrate is indicated suggest its versatility in addressing different medical conditions related to the nervous system, urogenital system, and other diseases. This broad range of therapeutic applications highlights the drug's potential to be used in diverse clinical settings.
With its long history of approval and the wide range of therapeutic indications, Fentanyl Citrate is an important drug in the pharmaceutical industry, offering valuable treatment options for patients dealing with various types of pain and related conditions. Its efficacy and versatility make it a significant asset in the field of biomedicine.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.