NGGT Reports Encouraging Results for NGGT002 in Treating PKU
NGGT Inc., is a biotechnology company at the clinical stage, leading advancements in gene therapy for rare genetic disorders. They recently shared promising new data regarding NGGT002, their gene therapy candidate currently in clinical trials for addressing Phenylketonuria (PKU).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Dr. Yiting Liu, VP of Translational Research at NGGT, expressed satisfaction with the investigator-initiated trial results, noting NGGT002's potential as a safe and effective treatment for PKU. "The promising outcomes over the initial 40-week period give us optimism for our Phase I/II clinical trials, which should further clarify NGGT002’s capability to address this rare and challenging disease sustainably," Liu stated.
Dr. Lixin Jiang, CEO and Co-Founder of NGGT, added, "The challenges of dietary management for PKU, along with the potential advantages of effectively reducing phenylalanine levels long-term, make these findings particularly exciting. NGGT002's notable safety and efficacy in the trial give us great anticipation for the results from our ongoing U.S. and China clinical trials."
These findings, showcased at the National PKU Alliance's biennial meeting in July, shed light on an investigator-initiated trial (IIT) in China, underscoring the safety and efficacy of NGGT002 for PKU treatment. PKU is a rare genetic disorder stemming from a mutation in the PAH gene, leading to increased blood phenylalanine levels, which progressively impair intellectual and behavioral functions and can cause skin rashes, movement disorders, and seizures. In the trial, five out of six patients on a high dose of NGGT002 had normal plasma phenylalanine levels after three weeks. The first IIT trial participant maintained normal plasma phenylalanine levels 40 weeks post-treatment. Initiated Phase I/II clinical trials in the U.S. (NCT06332807) and China (NCT06061614) aim to assess NGGT002's safety and efficacy in adult PKU patients, administering doses based on the effective levels demonstrated in the IIT study.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
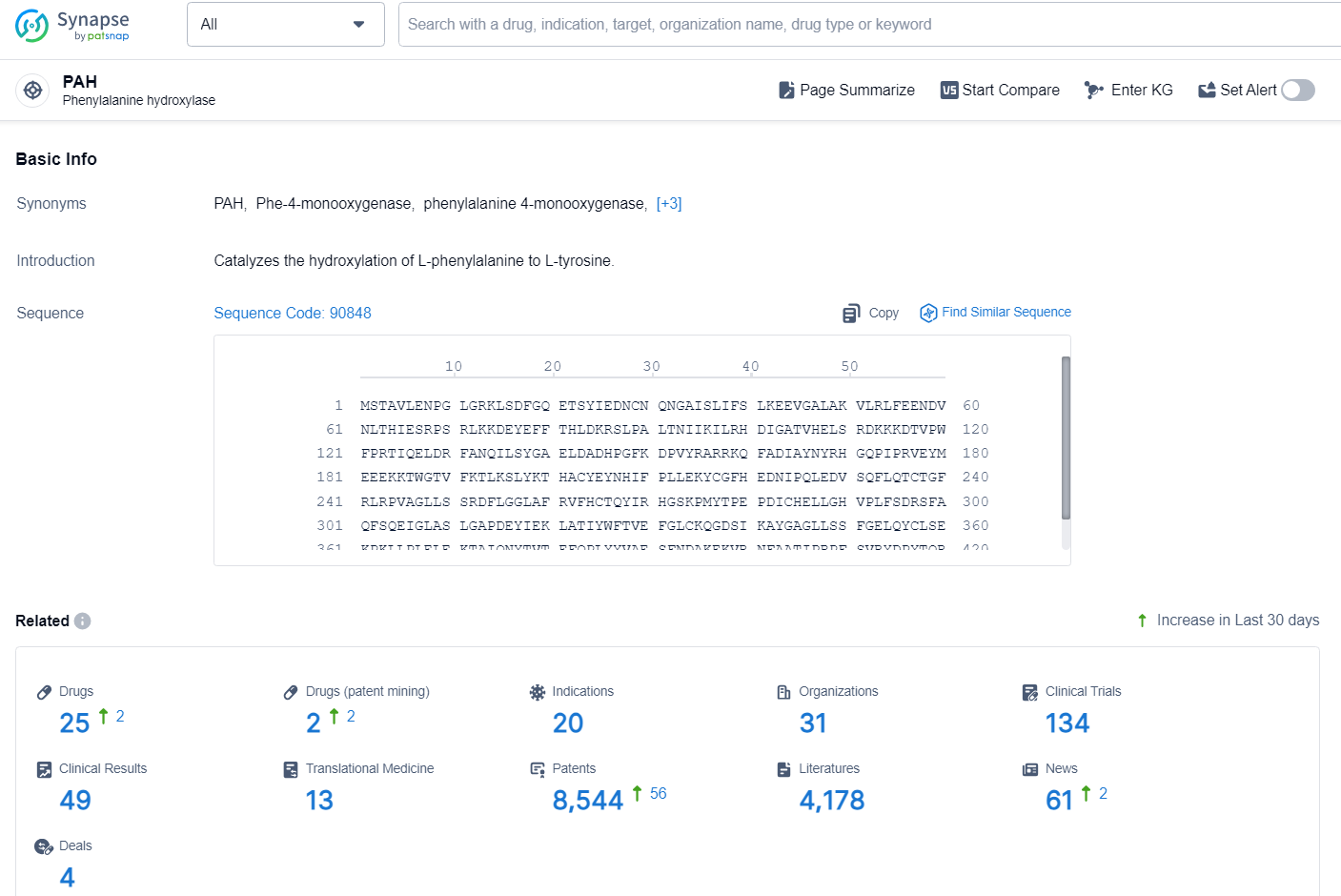
According to the data provided by the Synapse Chemical, As of November 13, 2024, there are 1 investigational drug for the PAH target, including 10 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 126 patents.
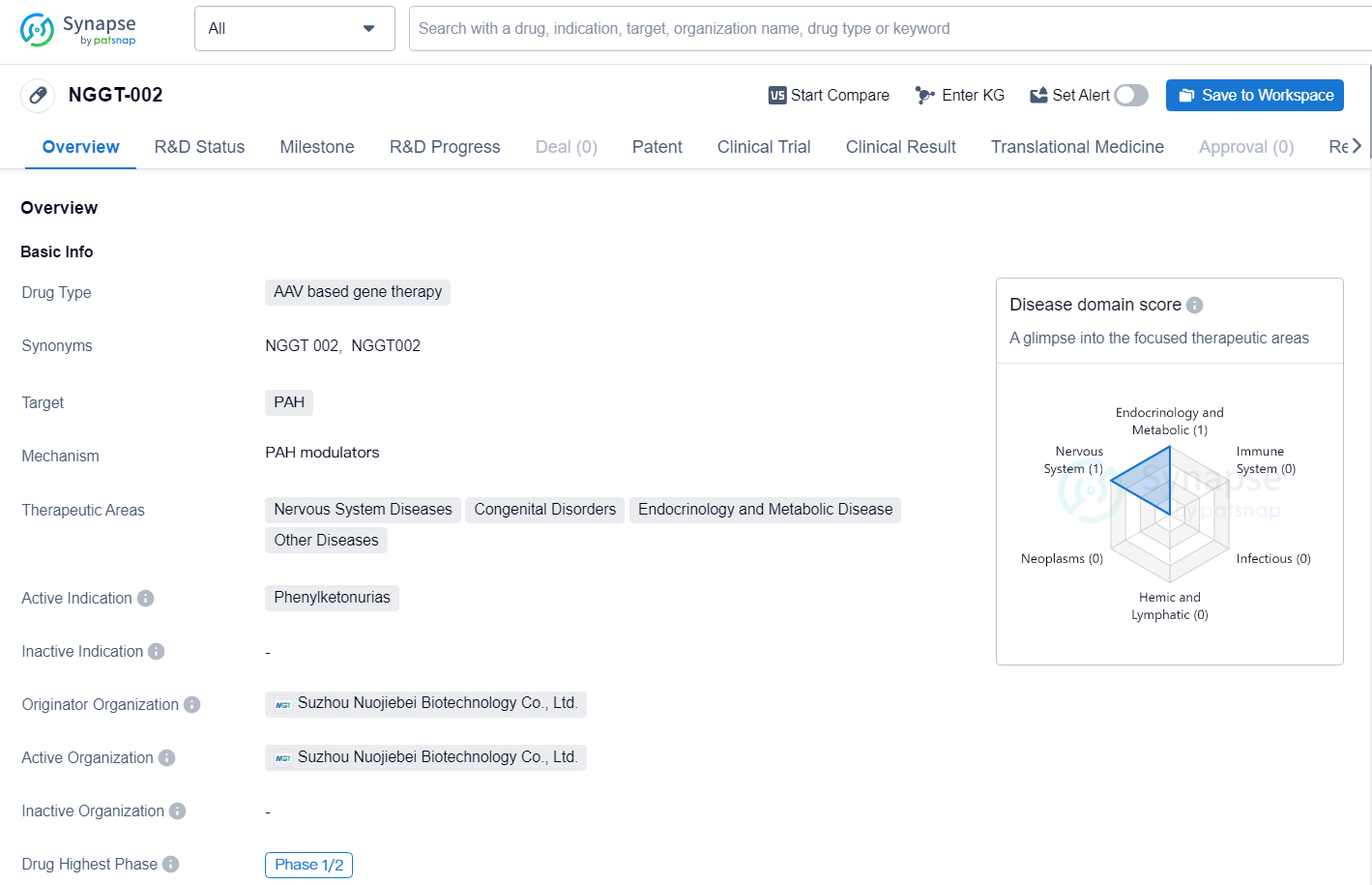
NGGT-002 is an AAV based gene therapy drug developed by Suzhou Nuojiebei Biotechnology Co., Ltd. The drug is designed to target PAH and is being developed for the treatment of Phenylketonurias, which falls under the therapeutic areas of Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, and Other Diseases. The drug has reached the highest phase of clinical development as Phase 1/2 globally, and has also achieved the same status in China.