Initial Dosing Reported in Phase 2 Study of FK-PC101, a Novel Personalized Cancer Immunotherapy by Theragent and CellVax
Theragent Inc., a full-service CDMO dedicated to the development of next-gen cell-based treatments, has reached an important achievement for itself and its partner, CellVax Therapeutics Inc. Recently, Theragent completed the initial production of an autologous cancer vaccine intended for administration to the first participant in CellVax Therapeutics’ randomized Phase 2 trial of FK-PC101.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are elated to be part of this success," remarked Dr. Yun Yen, the President and CEO of Theragent. "This accomplishment is the result of years of hard work, teamwork, and substantial perseverance from everyone involved. I extend my heartfelt gratitude to CellVax CEO Fernando Kreutz for trusting in our team; Theragent is honored to progress cell therapy products for CellVax and the larger healthcare sector. We were established precisely for this mission-to deliver revolutionary treatments to patients who need them," Dr. Yen continued.
CellVax has developed FK-PC101, an innovative personalized cancer immunotherapy designed for prostate cancer patients at high risk of recurrence after prostatectomy. It involves using the patient's own tumor cells, collected during surgery, which are then modified at the Theragent CGMP facility. The modified cells express Major Histocompatibility Complex (MHC) Class II on their surface, are irradiated to render them unable to replicate, and then administered as personalized immunotherapy.
Kreutz noted, "Despite advancements in radiation, surgeries, and other treatments, up to 30% of patients might still face recurrent prostate cancer post-prostatectomy. Additionally, for those with recurrence, the prevailing treatment options are salvage radiotherapy and/or androgen deprivation therapy (ADT), which can negatively impact patients' quality of life. FK-PC101 has the potential to delay, if not entirely prevent, the need for such interventions."
The CELLVX-230 trial is a multicenter, adaptive, Phase 2, randomized, open-label study focused on irradiated autologous cellular vaccines for men with high-risk prostate cancer following radical prostatectomy. Conducted in collaboration with the Society of Urologic Oncology Clinical Trials Consortium (SUO-CTC) and led by Principal Investigator Scott Eggener, MD, from the University of Chicago, Theragent manages the complete manufacturing process, distribution, and disposition of all clinical materials from its specialized, advanced CGMP cell therapy manufacturing facility in Arcadia, CA.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
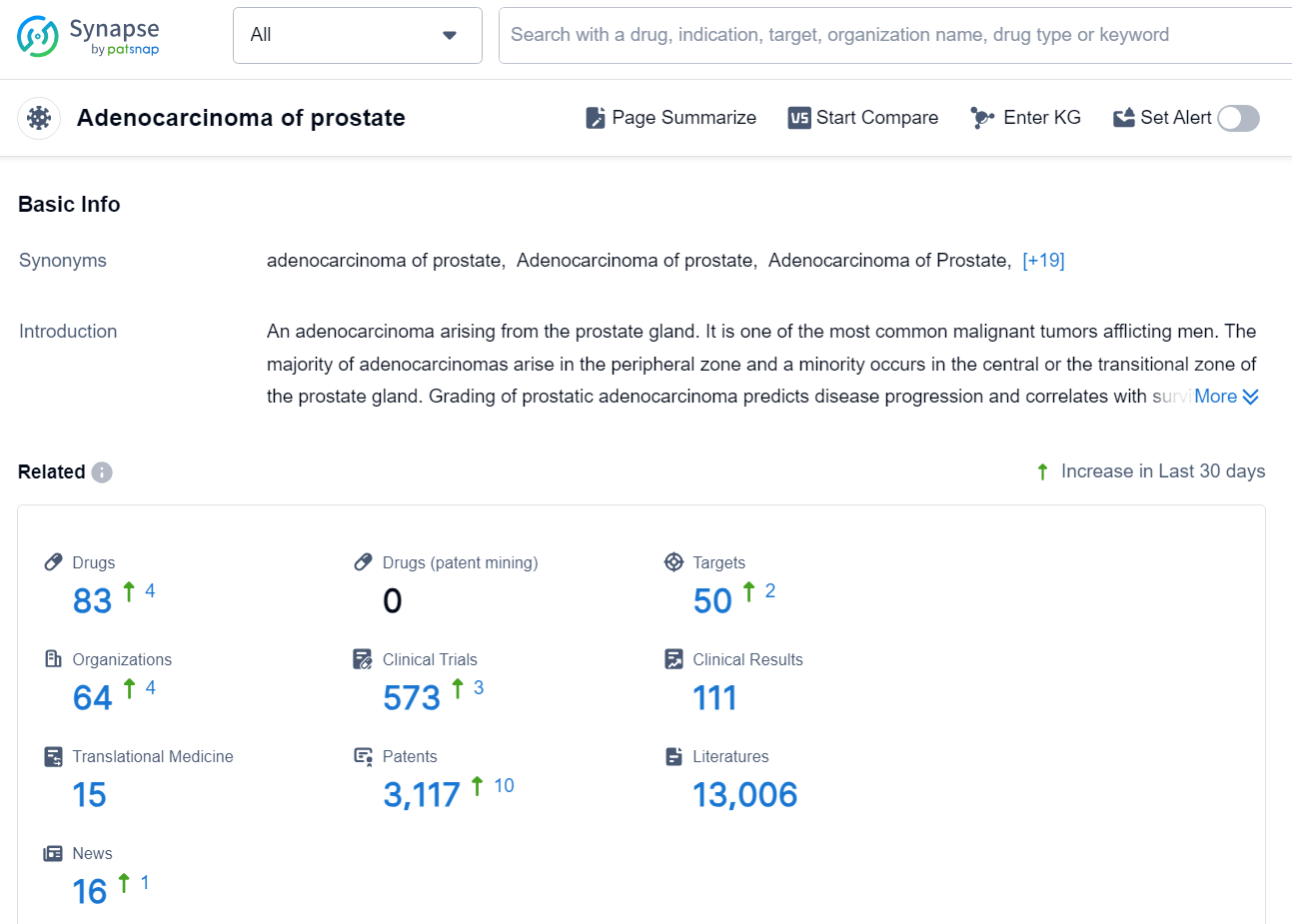
According to the data provided by the Synapse Database, As of November 13, 2024, there are 83 investigational drugs for the prostate, including 50 targets, 64 R&D institutions involved, with related clinical trials reaching 573, and as many as 3117 patents.
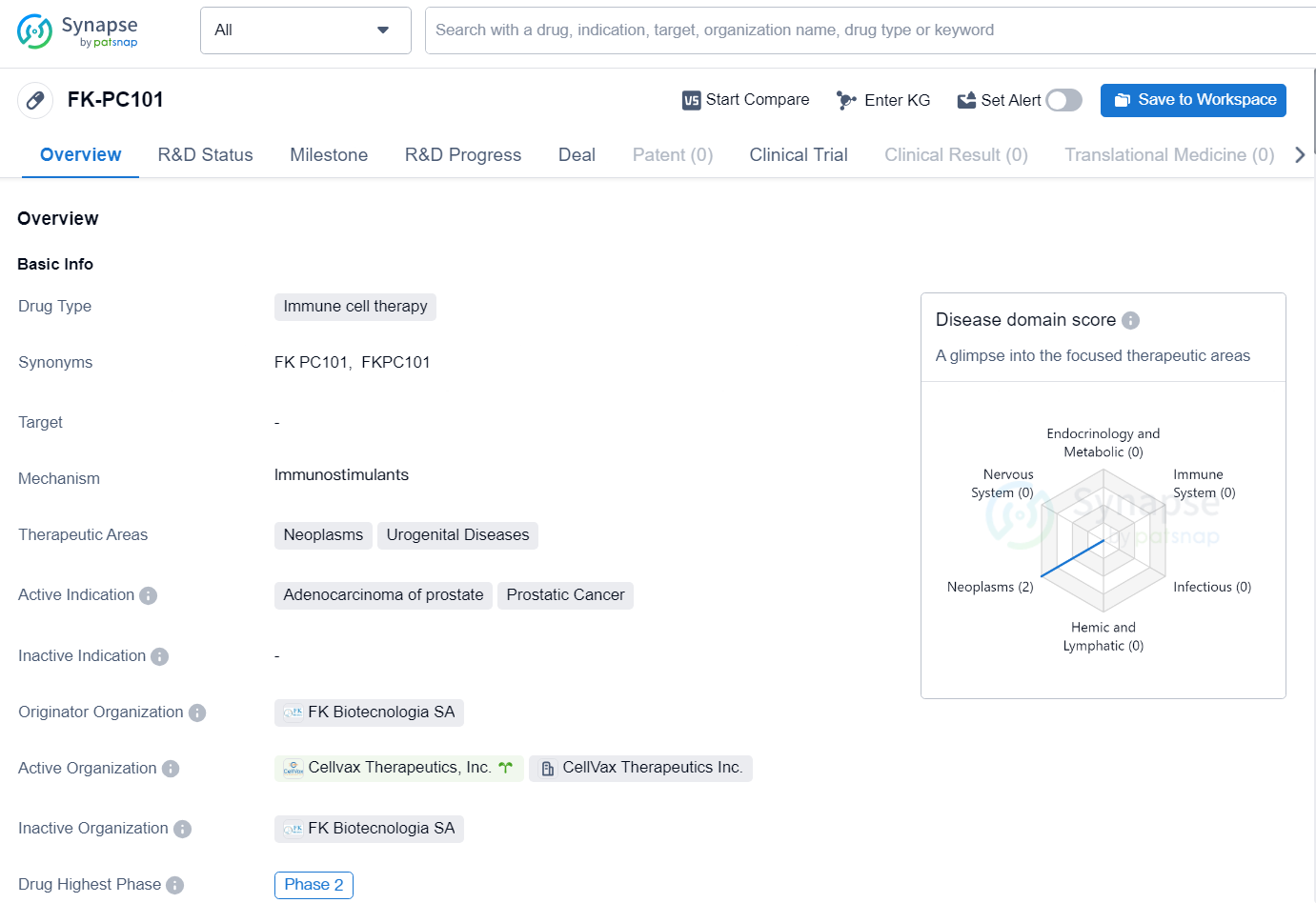
FK-PC101 is an immune cell therapy drug developed by FK Biotecnologia SA. The drug is currently in Phase 2 of its development and is primarily targeted towards the treatment of neoplasms and urogenital diseases. The active indications for FK-PC101 include adenocarcinoma of the prostate and prostatic cancer.