Exploring the Latest MSLN ADC Deal by Harbour Biomed Therapeutics: A Guide to Rapidly Accessing Transaction Insights
Harbour Biomed Therapeutics Ltd. and its wholly-owned subsidiary, Nona Biosciences, recently announced that they have signed an exclusive licensing agreement with Pfizer Inc. for the global clinical development and commercialization of the anti-mesothelin (MSLN) antibody-drug conjugate (ADC) HBM9033. According to the terms of the agreement, Harbour BioMed will receive an upfront and near-term payment of $53 million, as well as up to $1.05 billion in potential milestone payments. In addition, Harbour BioMed is also eligible to receive tiered royalties on net sales that range from high single-digit to high teens percentages.
About HBM9033
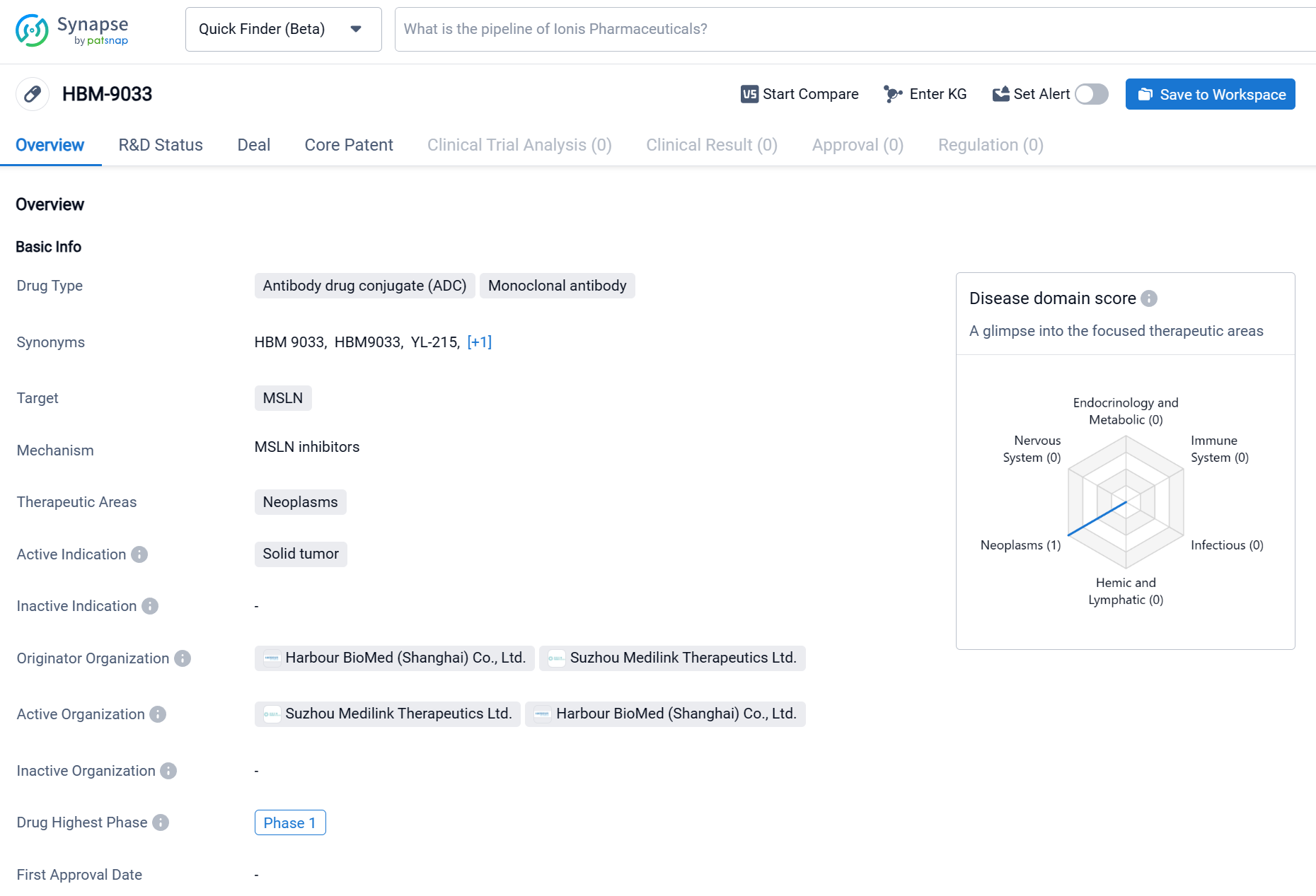
HBM9033 is an antibody drug conjugate (ADC) and monoclonal antibody that is being developed for the treatment of neoplasms, specifically solid tumors. HBM9033 is being developed by two organizations, Harbour BioMed (Shanghai) Co., Ltd. and Suzhou Medilink Therapeutics Ltd. Currently, HBM9033 is in the highest phase of clinical development, which is Phase 1. The specific therapeutic areas that HBM9033 is targeting are neoplasms, which refer to abnormal growths of tissue that can be cancerous. Click the image below to directly embark on the exploration journey with the HBM9033!
HBM9033 specifically targets MSLN, a tumor-associated antigen that is upregulated in a variety of solid tumors, including mesothelioma, ovarian cancer, lung cancer, breast cancer, and pancreatic cancer. This ADC utilizes a tumor-specific cleavable linker and a novel topoisomerase inhibitor payload to enhance stability and potency. Its unique antibody and linker-payload design have demonstrated outstanding efficacy and safety in preclinical studies.
About Harbour Biomed Therapeutics Ltd.
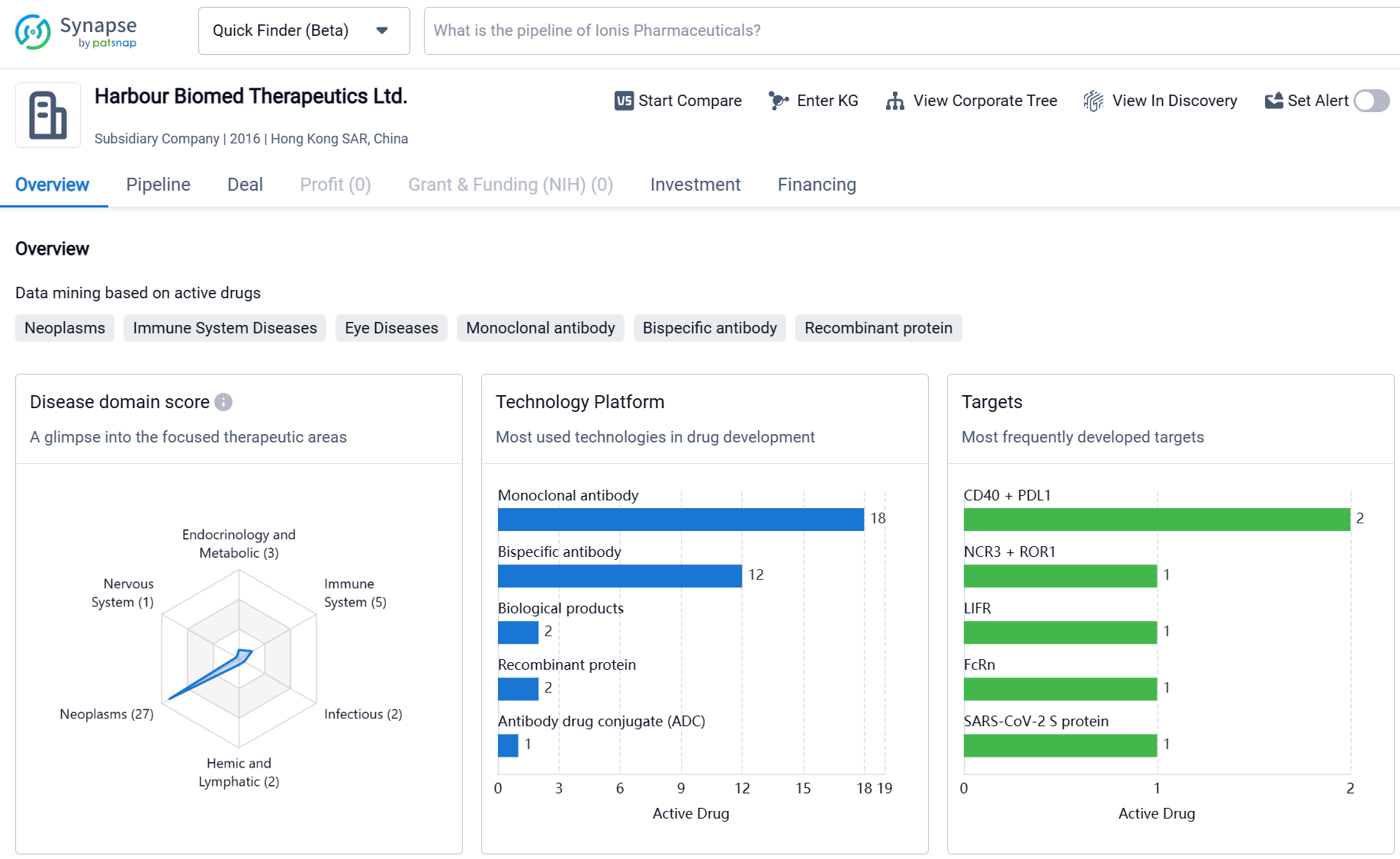
Harbour Biomed Therapeutics Ltd. is a relatively young company that has made significant progress in the development of drugs in various therapeutic areas. The company's focus on Neoplasms and Immune System Diseases reflects the growing demand for innovative treatments in these areas. The diverse range of molecular targets being pursued by the company indicates a commitment to exploring novel approaches for drug development. With a substantial number of drugs in the preclinical stage and several in advanced clinical stages, Harbour Biomed Therapeutics Ltd. has a promising pipeline that could potentially lead to the development of new therapies for patients in need.
By engaging in proprietary research and diversified collaboration, Harbour BioMed has rapidly expanded its new drug research and development pipeline, which now boasts a robust lineup including monoclonal antibodies, bispecific antibodies, and antibody-drug conjugates (ADCs). From the perspective of immune pathways, the pipeline covers direct-killing agents, T cell recruiters, T/NK cell activators, NK cell recruiters, and therapies that relieve immunosuppression in the tumor microenvironment, among others.
How to get the latest progress on drug deals?
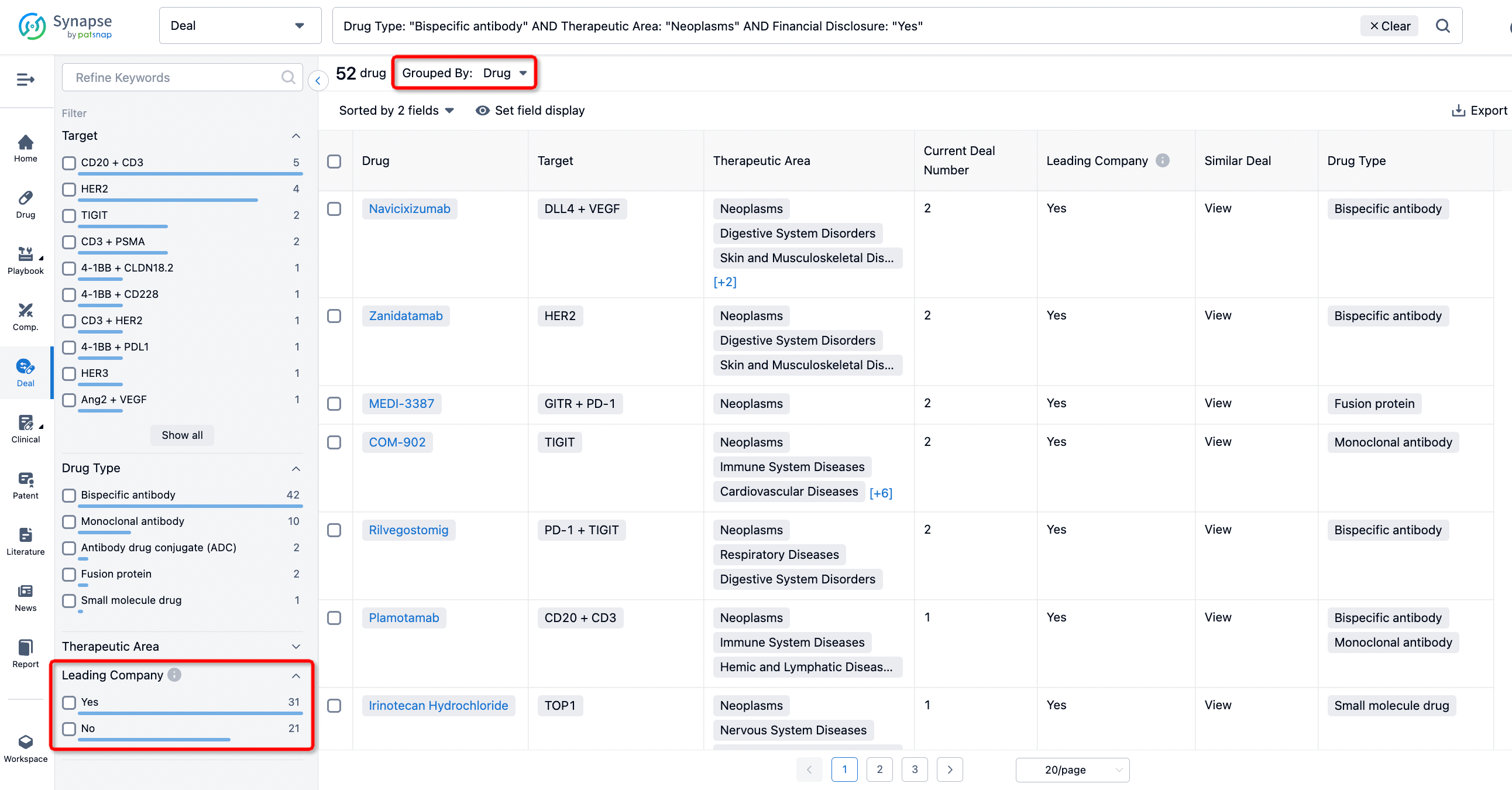
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
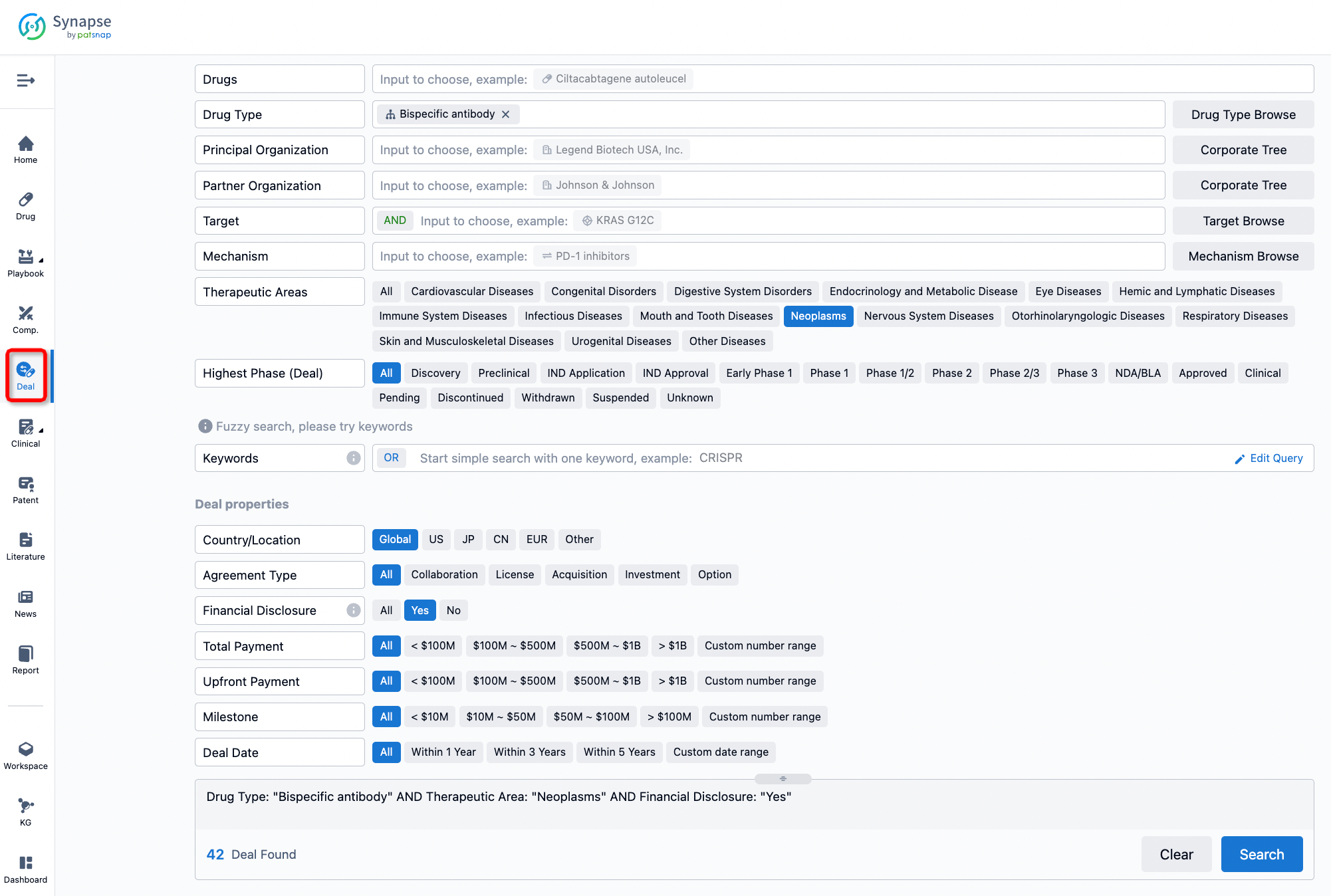
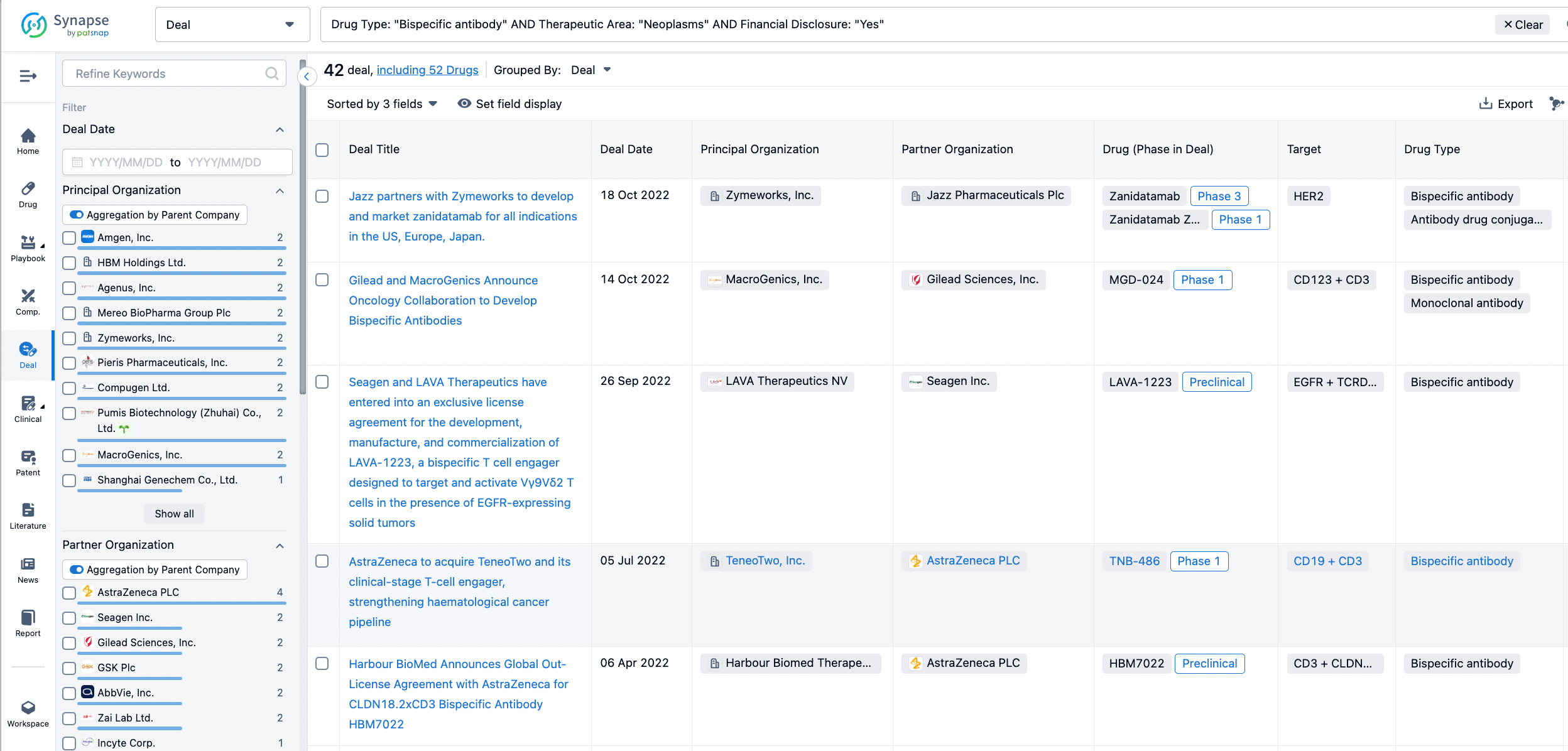
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
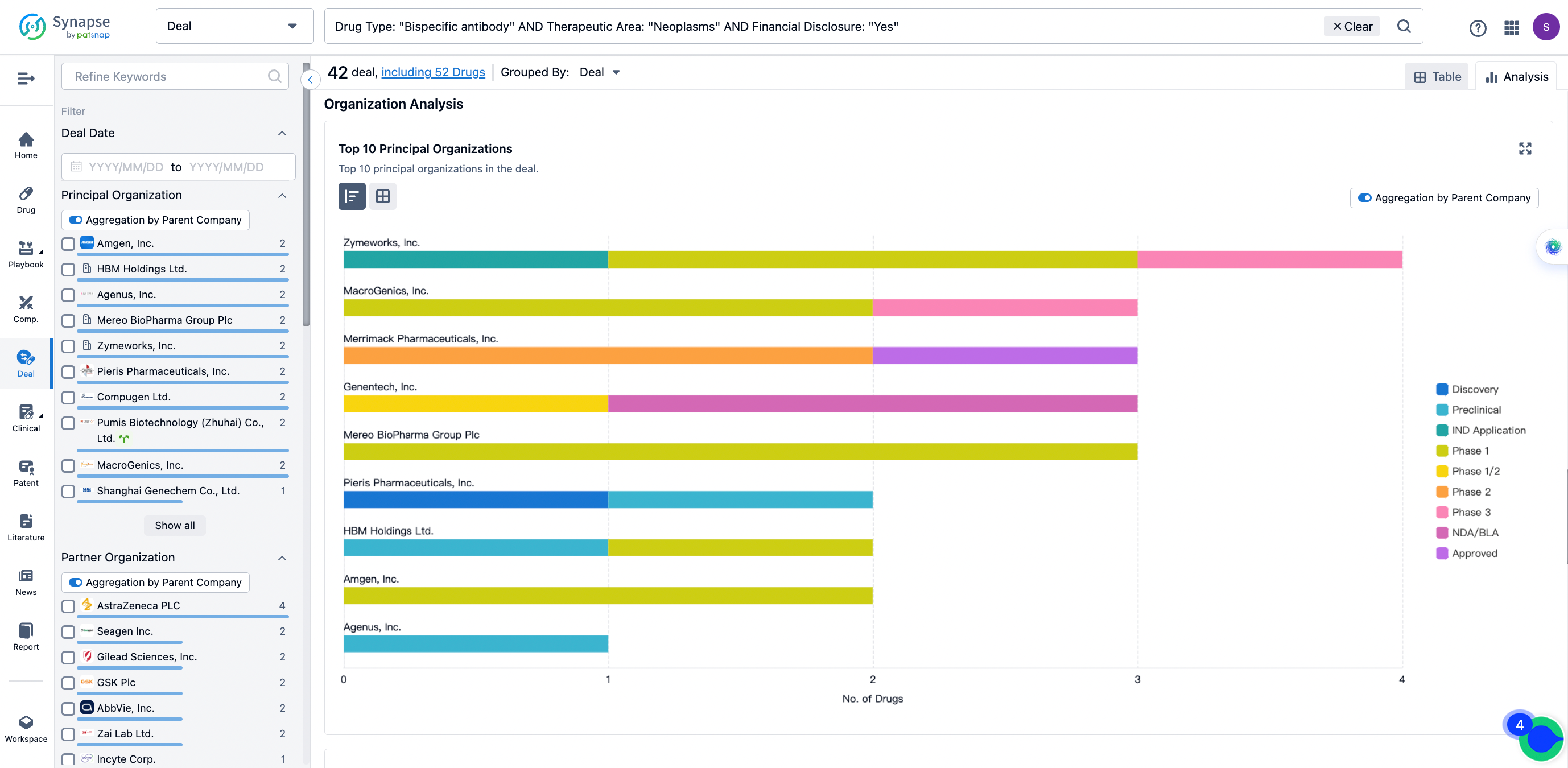
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
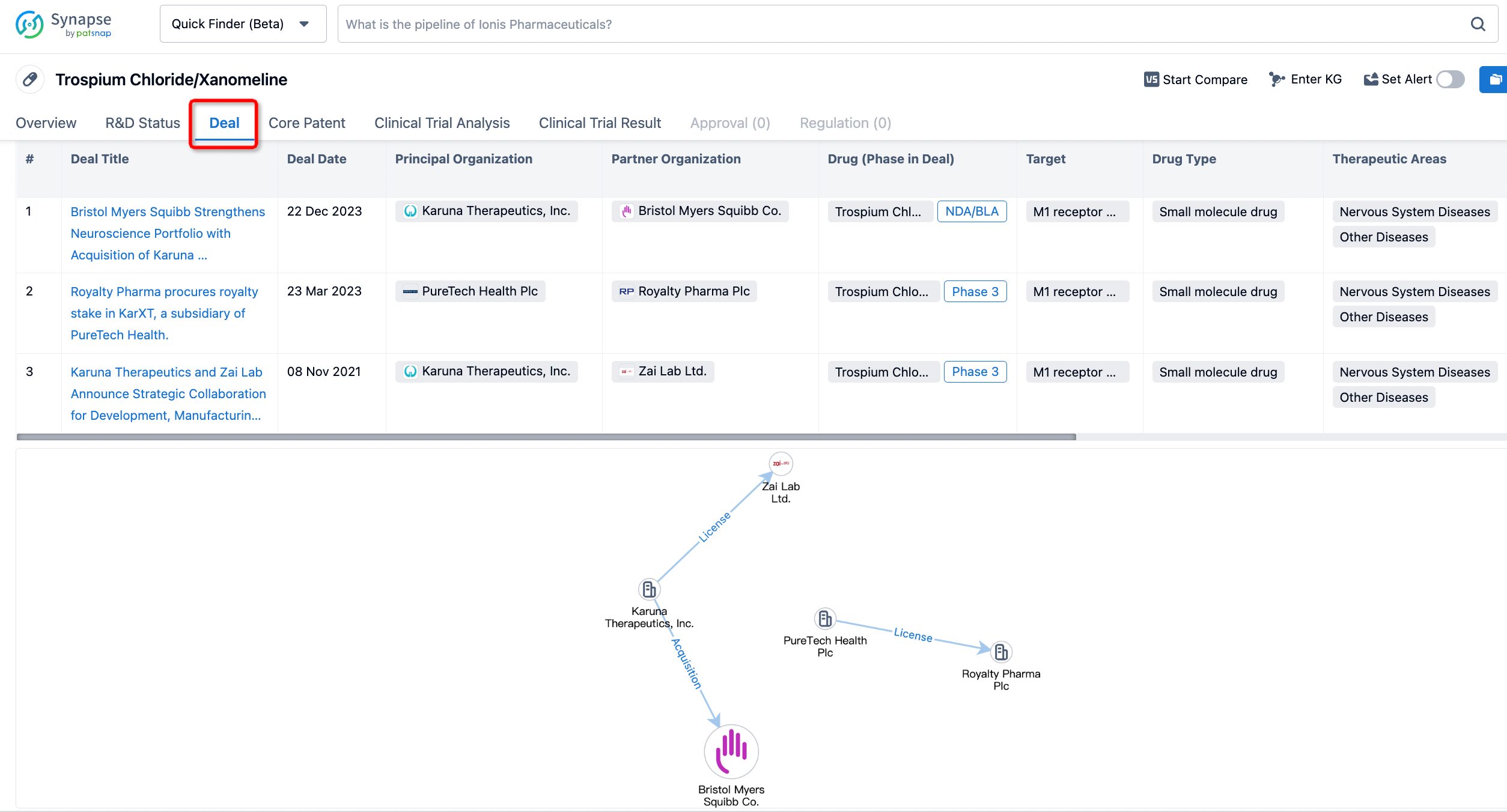
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
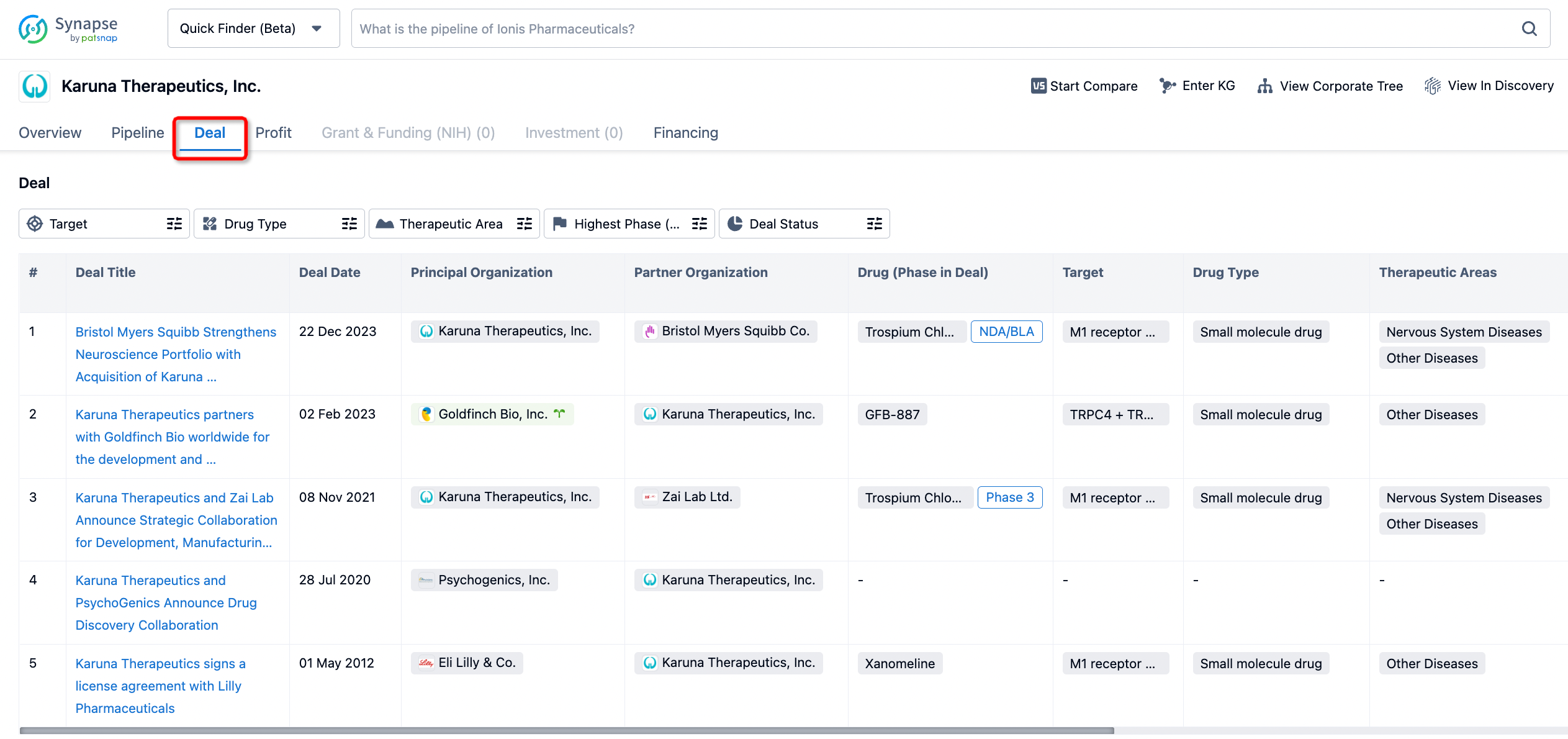
Click on the image below to embark on a brand new journey of drug discovery!