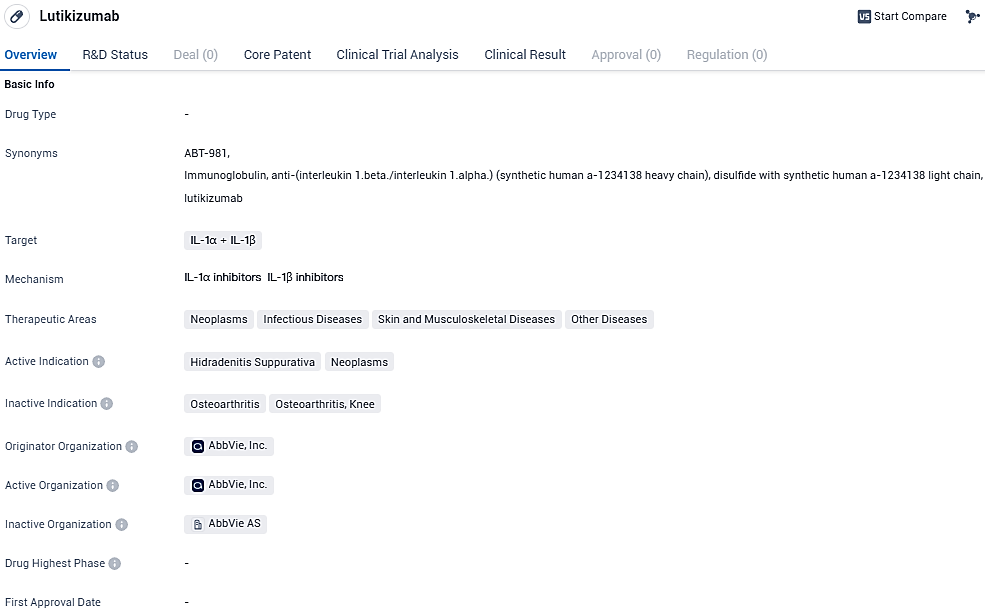
Lutikizumab Shows Promise in Phase 2 Trial for Moderate to Severe Hidradenitis Suppurativa, Advances to Next Study Phase
Pharmaceutical company AbbVie declared findings from a Phase 2 clinical trial, which indicate that patients suffering from moderate to severe hidradenitis suppurativa (HS) and unresponsive to prior anti-TNF treatments, experienced better outcomes when administered 300 mg doses of lutikizumab (ABT-981), either biweekly or every week. These individuals exhibited greater rates of improvement compared to those receiving a placebo, particularly regarding the primary goal of attaining a HS Clinical Response by the 16th week of treatment. Following the assessment of these outcomes, AbbVie plans to progress the investigation of lutikizumab for HS into a Phase 3 trial.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"At AbbVie, we are at the forefront of developing novel therapies for those suffering from hidradenitis suppurativa, an often neglected and inadequately served demographic experiencing considerable distress," commented Roopal Thakkar, M.D., the company's senior vice president and chief medical officer of global therapeutics. "With over two decades of accumulated knowledge in treating immune-mediated conditions, we are dedicated to the progression of our Phase 3 clinical trials investigating lutikizumab for HS."
The research undertaken was a 16-week, Phase 2 clinical trial which was randomized, double-blind, with placebo control, covering multiple centers, and involving a dose range study. It focused on assessing the safety and effectiveness of lutikizumab in 153 adults with moderate to severe HS who did not respond adequately to previous anti-TNF treatment.
The vast majority of participants were dealing with advanced Hurley Stage 3 HS at the initiation of the study. This stage is associated with extensive scarring, the presence of persistent lesions, and the formation of sinus tracts. At the start of the trial, participants were divided into groups to receive varying doses of lutikizumab subcutaneously or a placebo. The primary goal of the study was to observe a 50% reduction in the Hidradenitis Suppurativa Clinical Response (HiSCR) score by the 16th week, with a secondary focus on a 30% reduction in skin pain as measured by the Numeric Rating Scale (NRS30) in participants by week 16.
"HS carries a significant burden for those affected, including prolonged periods prior to diagnosis, intense pain, impairment, social withdrawal, and a general decrease in life quality," mentioned Alexa B. Kimball, M.D., MPH, a participant in the study, hailing from Beth Israel Deaconess Medical Center in Boston, and a Dermatology Professor at Harvard Medical School. "These promising findings enhance our comprehension of lutikizumab's potential benefits for HS patients and aid in the pursuit of further therapeutic avenues for individuals battling with this condition."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
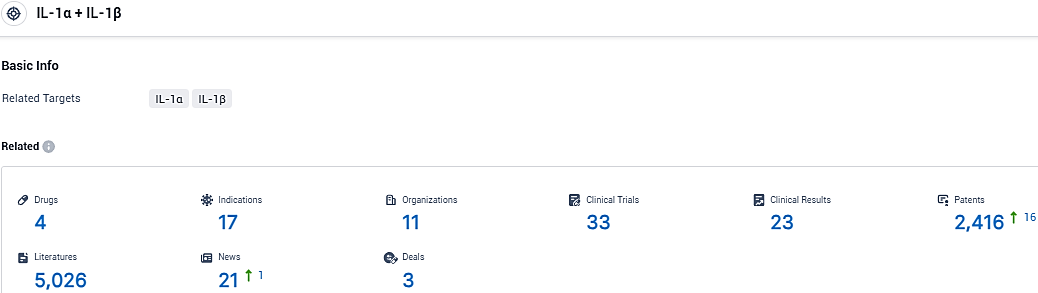
According to the data provided by the Synapse Database, As of January 13, 2024, there are 4 investigational drugs for the IL-1α and IL-1β tagets, including 17 indications, 11 R&D institutions involved, with related clinical trials reaching 33, and as many as 2416 patents.
Lutikizumab (ABT-981) is a dual-variable-domain interleukin (IL) 1α/1β antagonist being investigated in several immune-mediated diseases, including HS and ulcerative colitis. Studies have shown IL 1α and 1β are elevated in HS lesions.8 Lutikizumab is an investigational agent and is not approved by regulatory authorities. Safety and efficacy have not been established.