Igrelimogene litadenorepvec: A Quick Look at Its R&D Progress and Clinical Results from the 2023 ESMO_IO
On 06 Dec 2023, the latest clinical findings of Igrelimogene litadenorepvec were unveiled at the 2023 ESMO_IO, demonstrating its potential benefit and setting the stage for subsequent investigations.
Igrelimogene litadenorepvec's R&D Progress
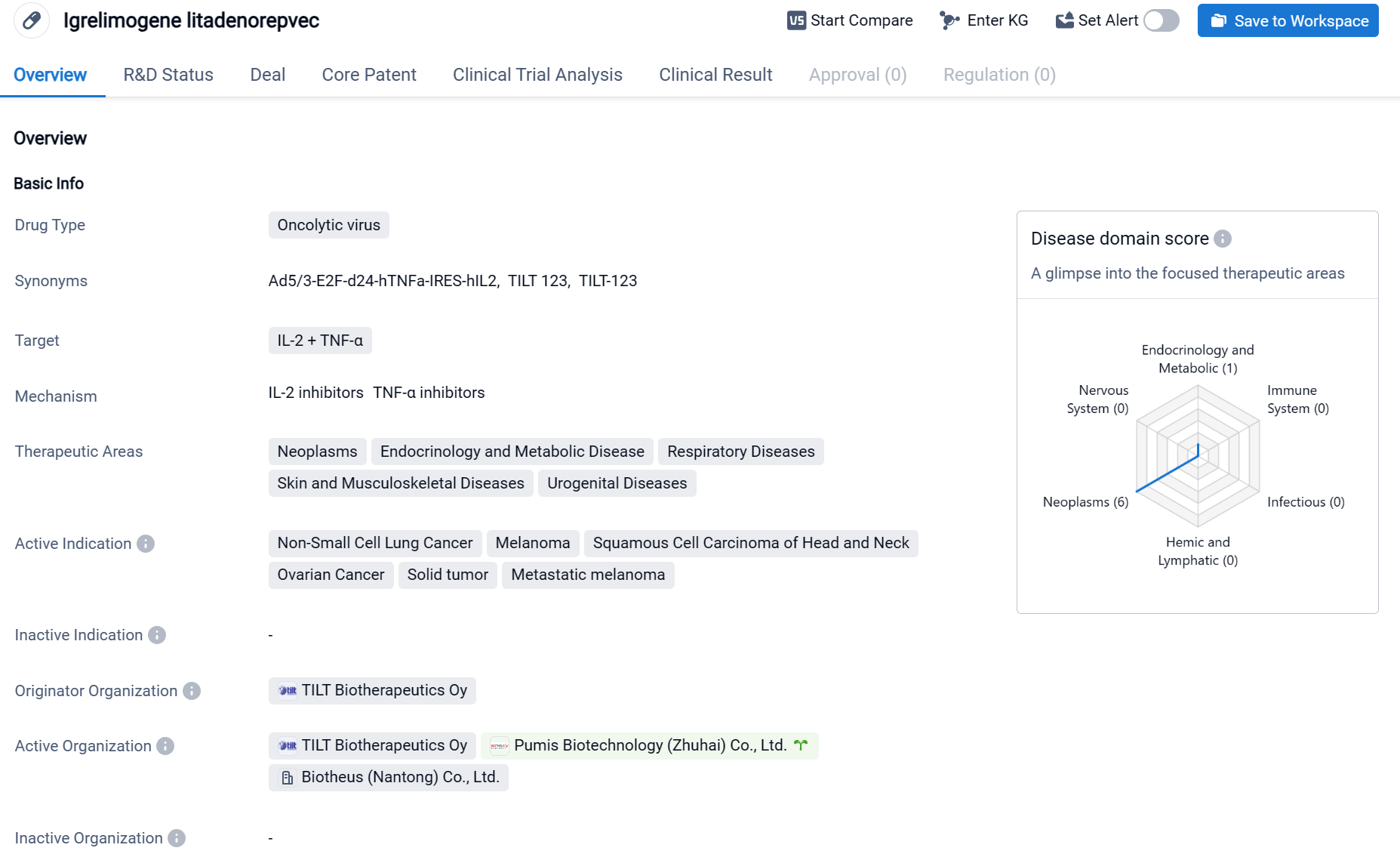
Igrelimogene litadenorepvec, also called TILT-123, is an oncolytic virus drug that targets IL-2 and TNF-α. It is being developed by TILT Biotherapeutics Oy and has reached Phase 1 of clinical trials globally. The drug is primarily focused on treating various types of cancers, including Non-Small Cell Lung Cancer, Melanoma, Squamous Cell Carcinoma of Head and Neck, Ovarian Cancer, Solid tumor, and Metastatic melanoma. These therapeutic areas cover a wide range of neoplasms, endocrinology and metabolic diseases, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases.
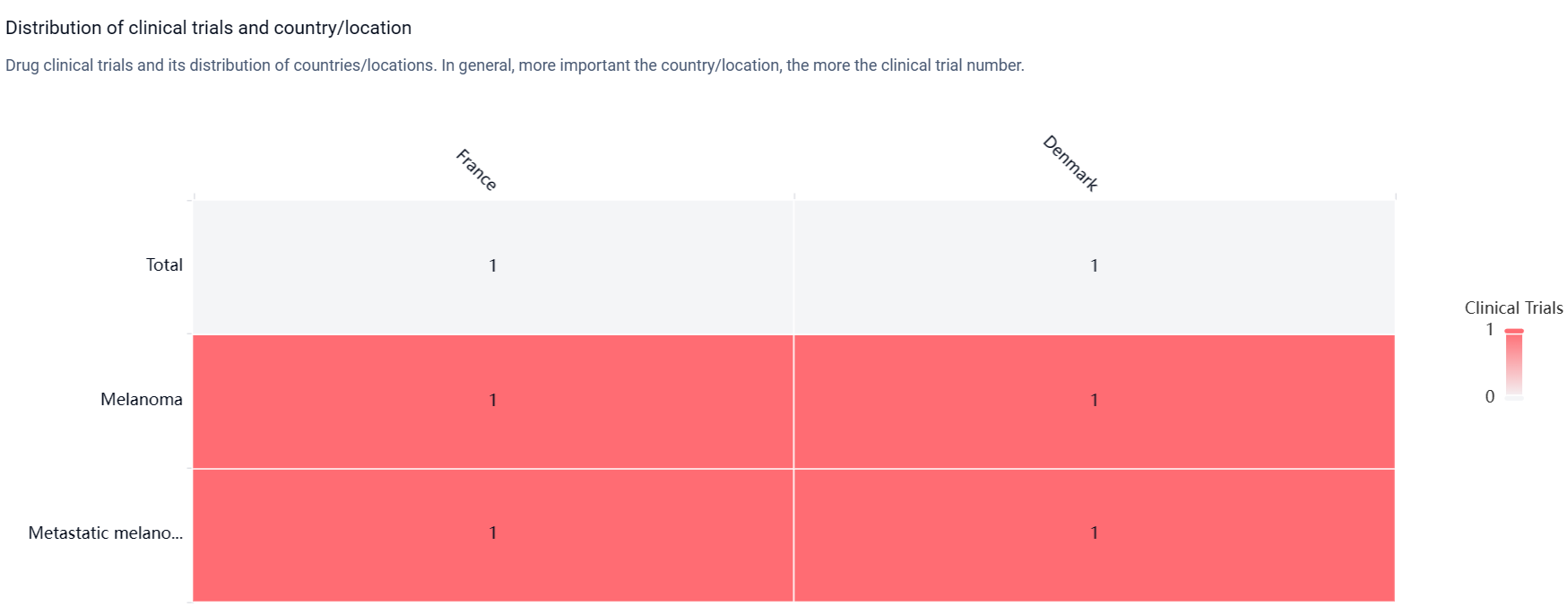
According to the Patsnap Synapse, Igrelimogene litadenorepvec is in Phase 1 of clinical development. And the clinical trial distributions for Igrelimogene litadenorepvec are primarily in France and Denmark. The key indication is Metastatic melanoma.
Detailed Clinical Result of Igrelimogene litadenorepvec
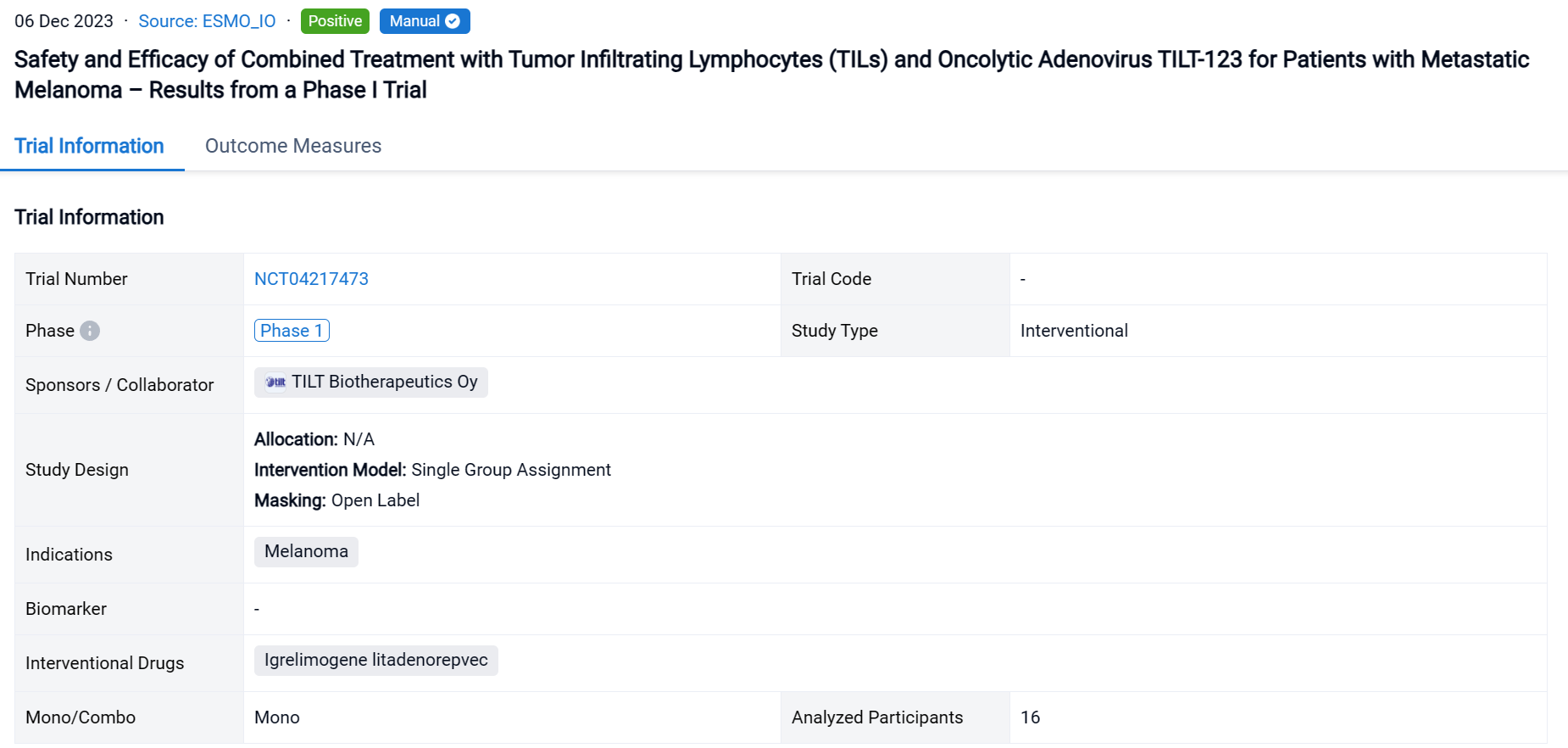
This single group assignment, open-labeled clinical trial (NCT04217473) was aimed to evaluate the safety and efficacy of combined treatment with tumor infiltrating lymphocytes (TILs) and oncolytic adenovirus TILT-123 for patients with metastatic melanoma.
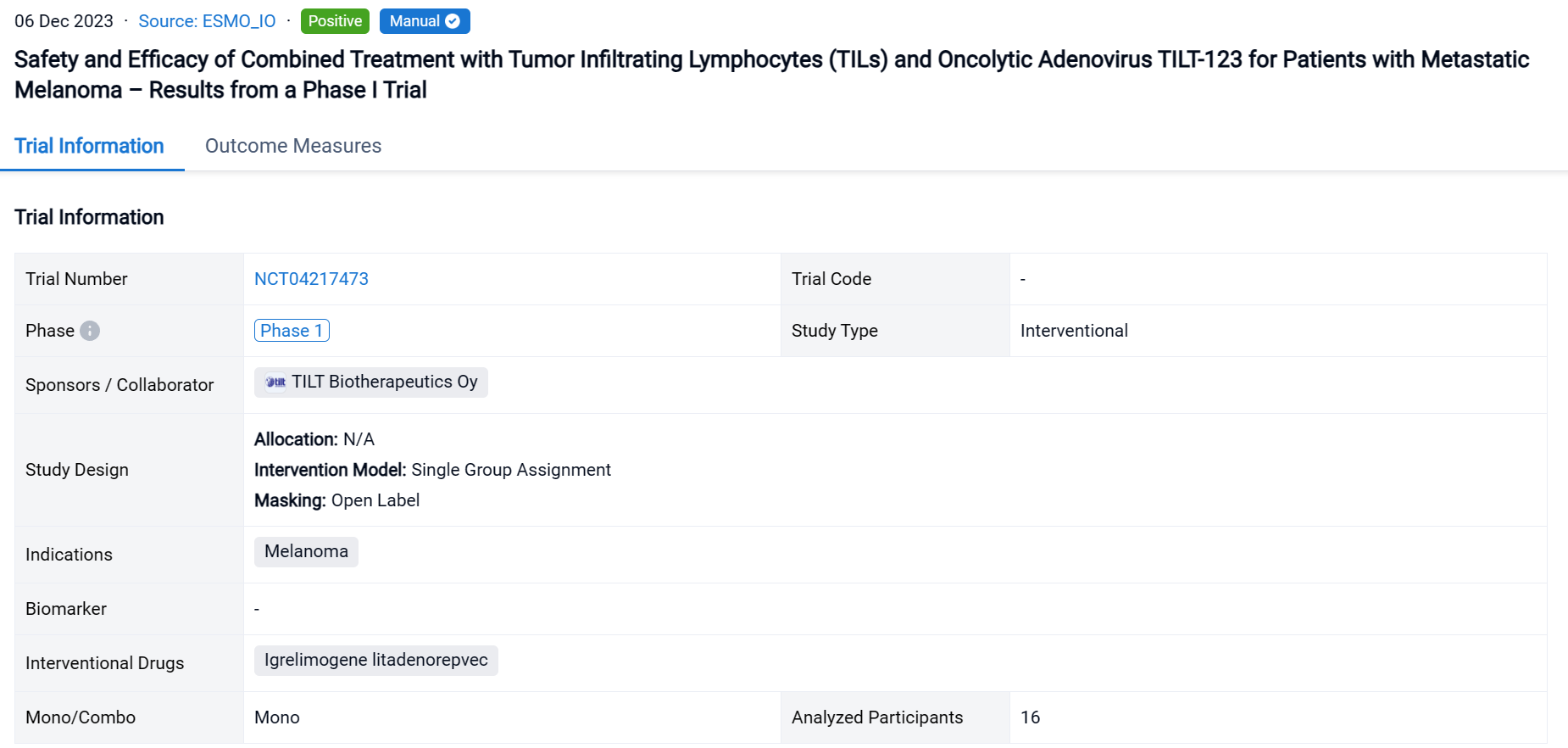
In this study, patients with stage IV melanoma were treated with multiple intravenous and intratumoral injections of TILT-123 and a one- or two-time treatment with TILs. TILs were grown from resected tumor tissue and administered without pre- or post-conditioning treatment regimens. The primary endpoint of the study was safety of TILT-123. Secondary endpoints included safety and efficacy of TILT-123 in combination with TILs.
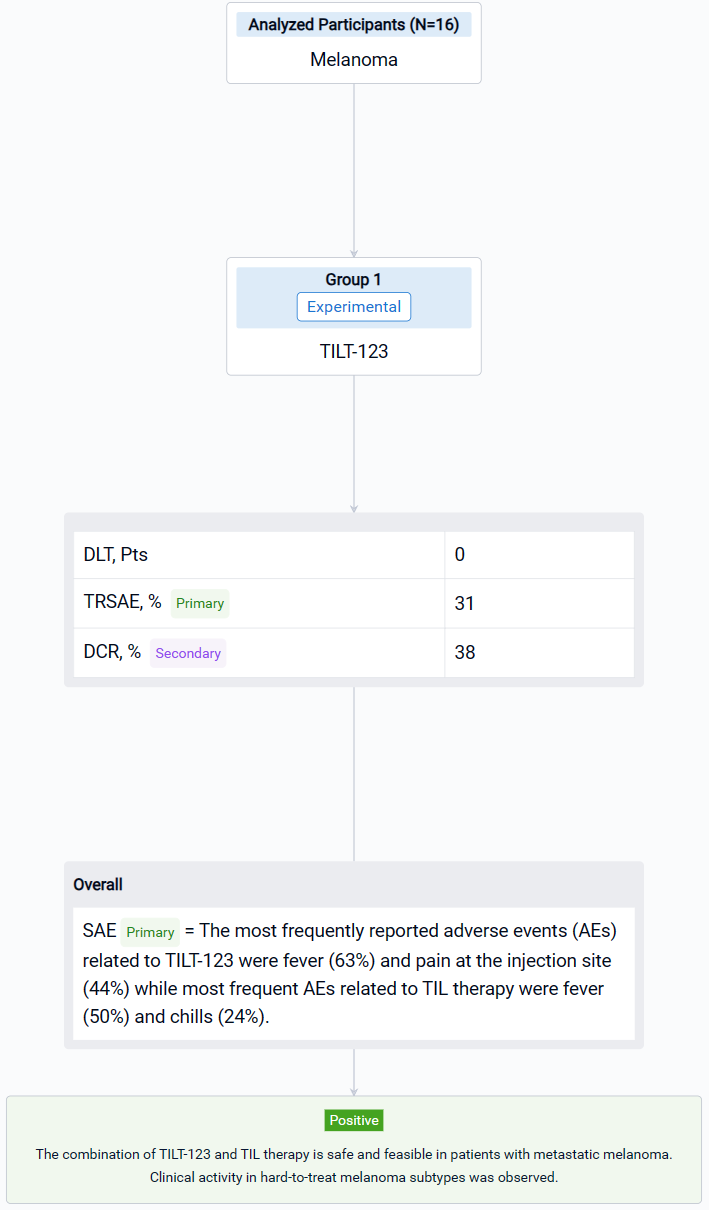
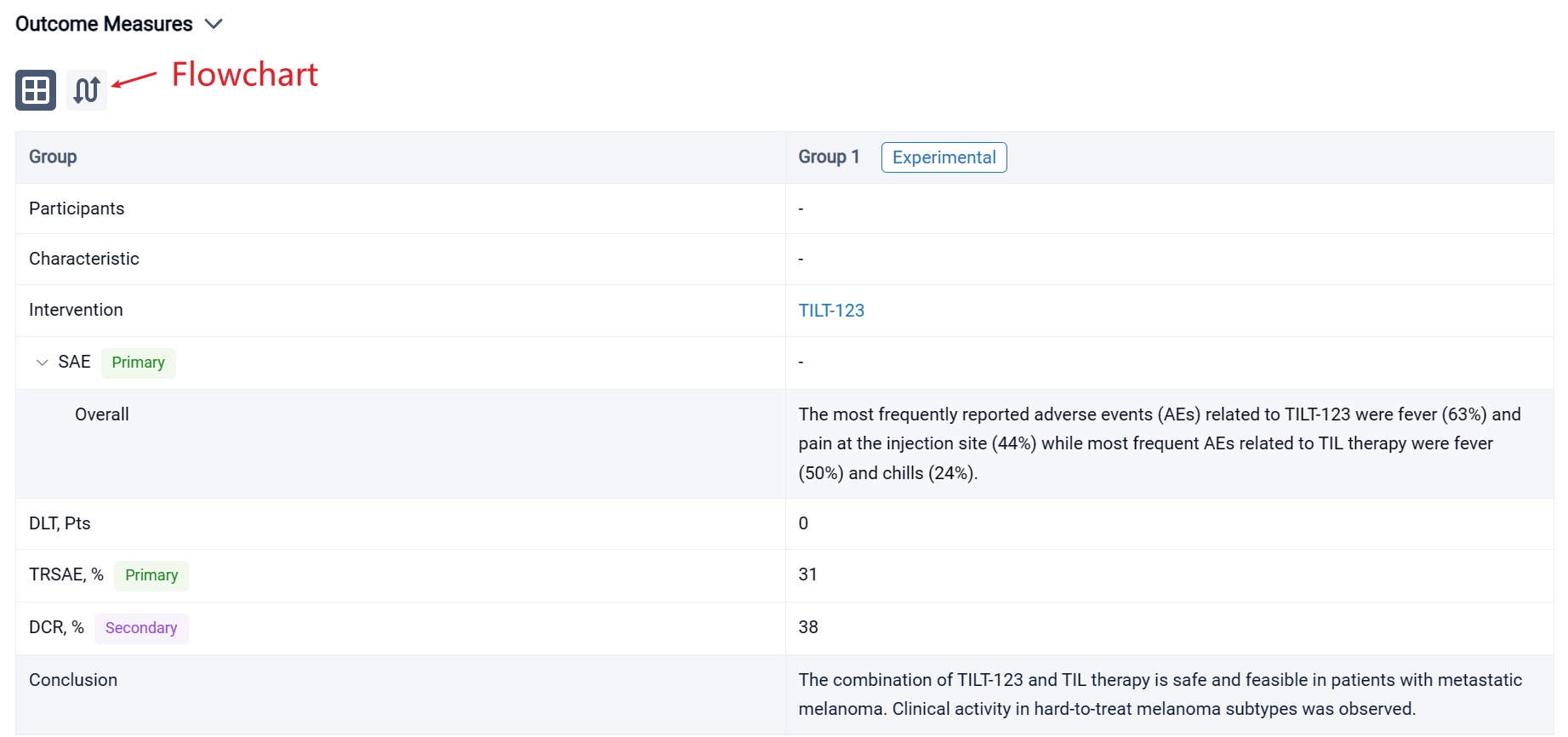
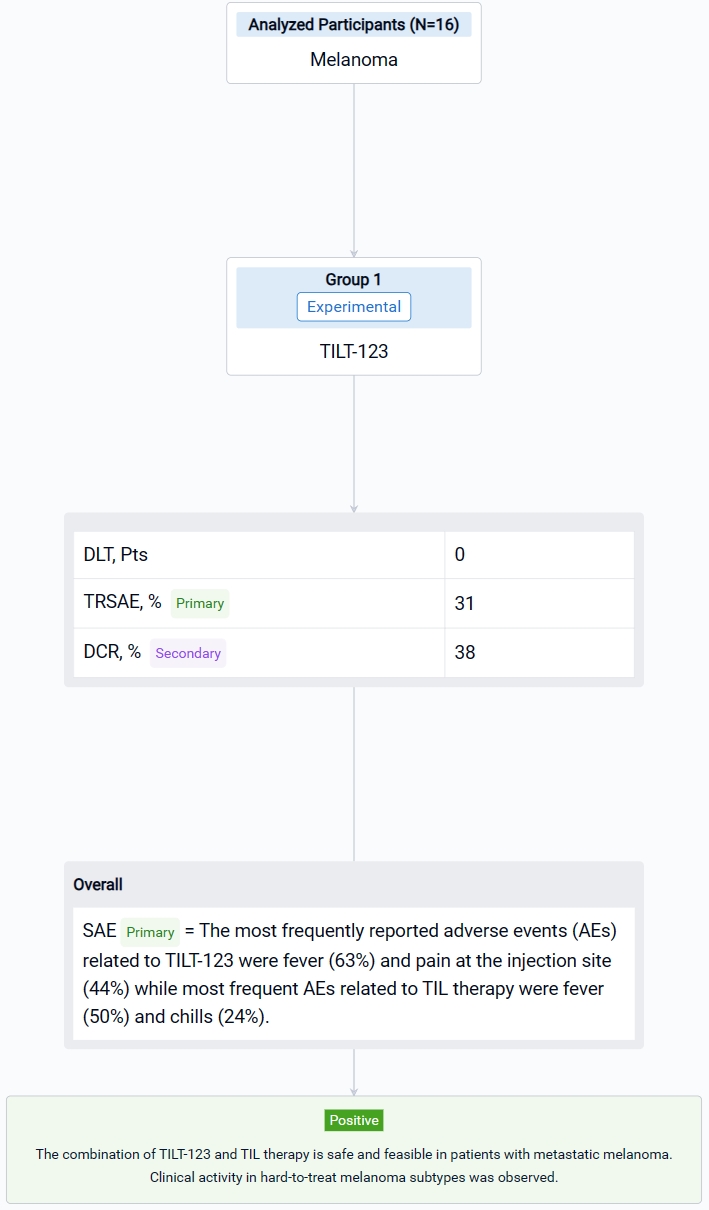
The result showed that sixteen patients with CPI resistant progressive cutaneous (7), mucosal (5) and uveal (4) metastatic melanoma were treated. Median age was 65.5 years (25-75). The most frequently reported adverse events (AEs) related to TILT-123 were fever (63%) and pain at the injection site (44%) while most frequent AEs related to TIL therapy were fever (50%) and chills (24%). No dose-limiting toxicity was observed and the combination of TILT-123 and TIL therapy did not increase the severity of AEs. 31% of patients experienced treatment related serious adverse events. In d78 imaging, RECIST1.1 responses were seen in two patients while disease control rate was 38 %. Responders included one patient (cutaneous) with an ongoing partial response and one patient (mucosal) with a durable complete response. Further, two patients (uveal and cutaneous) had long lasting stable disease (> 10 months). PET evaluation on d78 revealed disease control in 6/13 evaluable patients, including 4 partial or minor responses. On d36, after 4 injections of TILT-123 and before TILs, 4 partial or minor responses were seen in PET.
It can be concluded that the combination of TILT-123 and TIL therapy is safe and feasible in patients with metastatic melanoma. Clinical activity in hard-to-treat melanoma subtypes was observed.
How to Easily View the Clinical Results Using Synapse Database?
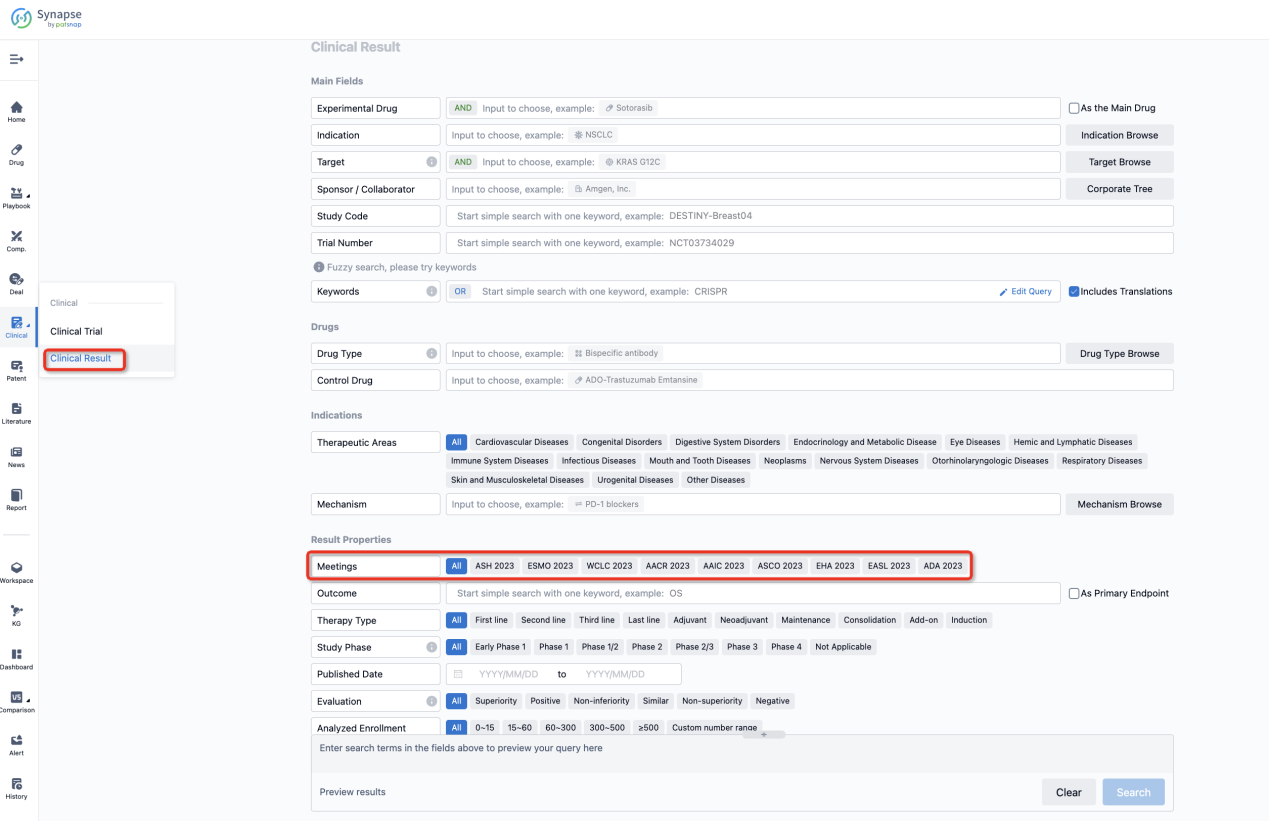
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
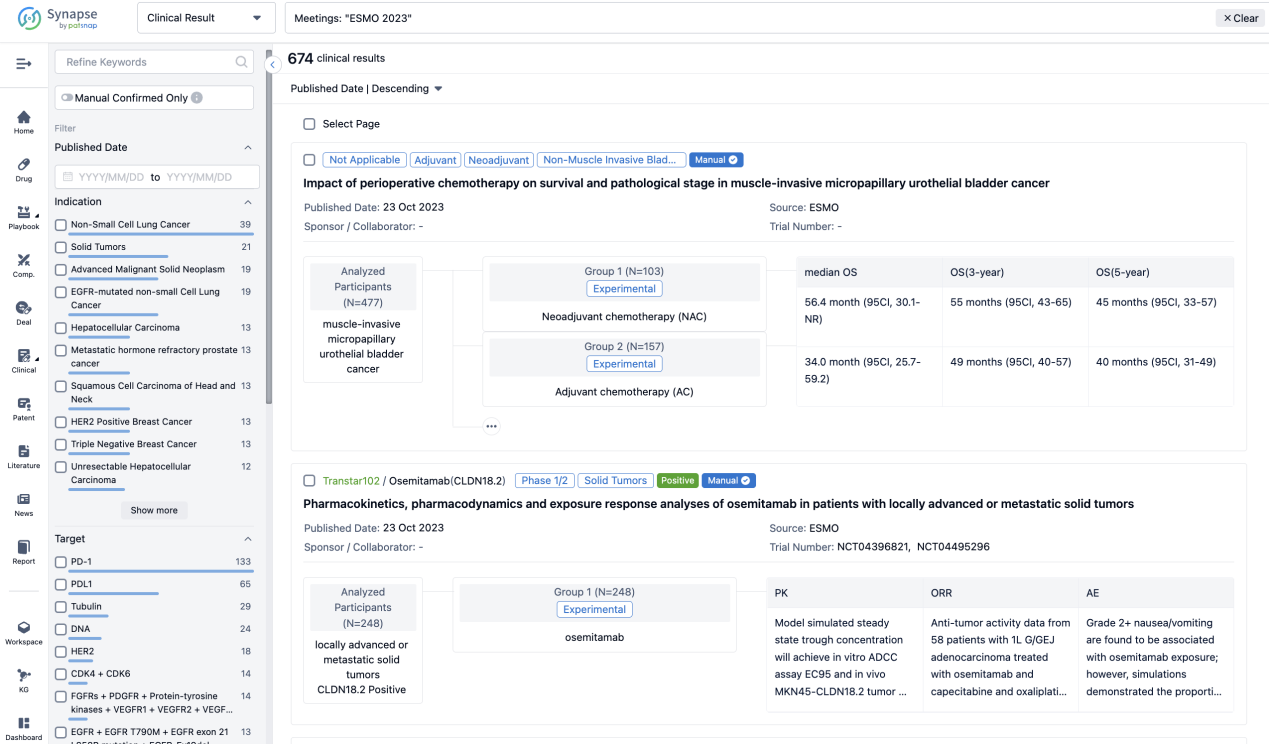
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
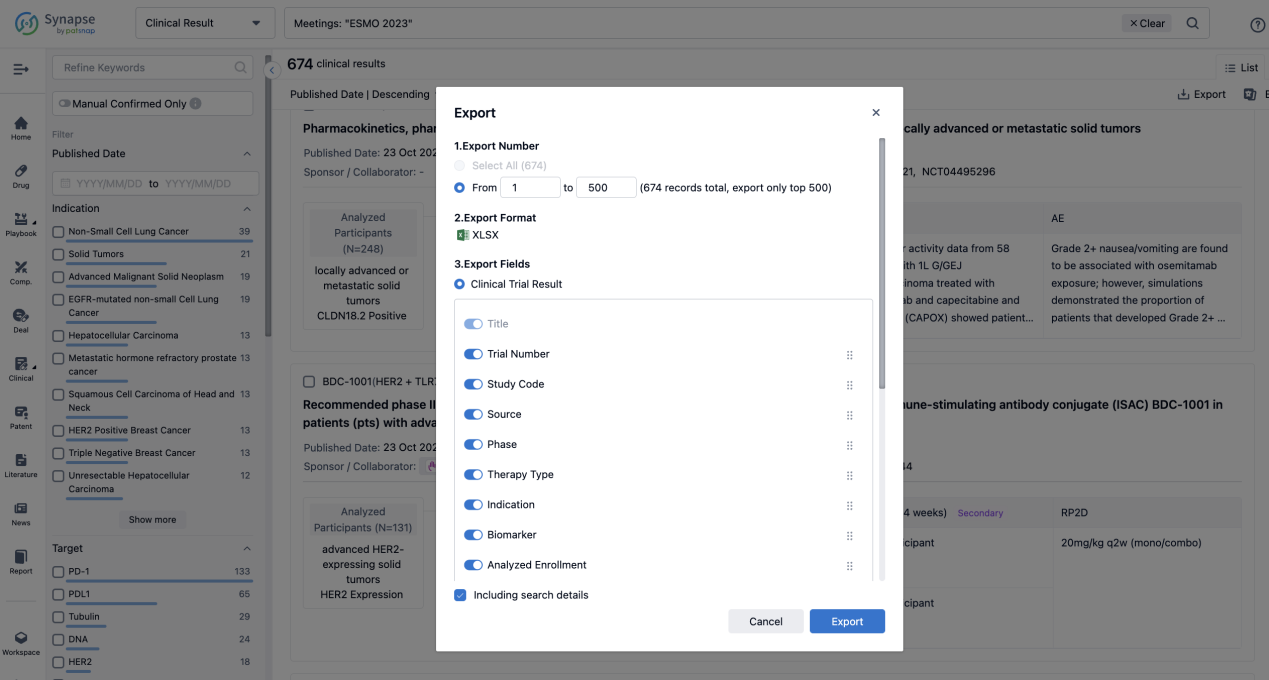
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!