Exploring the Latest TSLP monoclonal antibody Deal by Aiolos Bio: A Guide to Rapidly Accessing Transaction Insights
On January 9, 2024, GSK Plc and Aiolos Bio, Inc. jointly announced that they have reached an agreement. According to the agreement, GSK will acquire Aiolos with an upfront payment of $1 billion and potential regulatory milestone payments of up to $400 million. This acquisition grants GSK access to Aiolos's key product, AIO-001, a potential best-in-class, long-acting monoclonal antibody against thymic stromal lymphopoietin (TSLP). AIO-001 (SHR-1905) was developed by Hengrui Medicine. In August 2023, Aiolos entered into an agreement with Hengrui, paying an upfront payment of $21.5 million (plus over $1 billion in potential milestones) for the exclusive global rights to develop and commercialize the drug outside of Greater China, while Hengrui retained commercialization rights within the Greater China market. After the transaction is completed, GSK will be responsible for continuing to make milestone payments and tiered royalties to Hengrui. By rough estimates, within five months, the value of this pipeline has increased nearly 40-fold based on the upfront payment alone.
About AIO-001
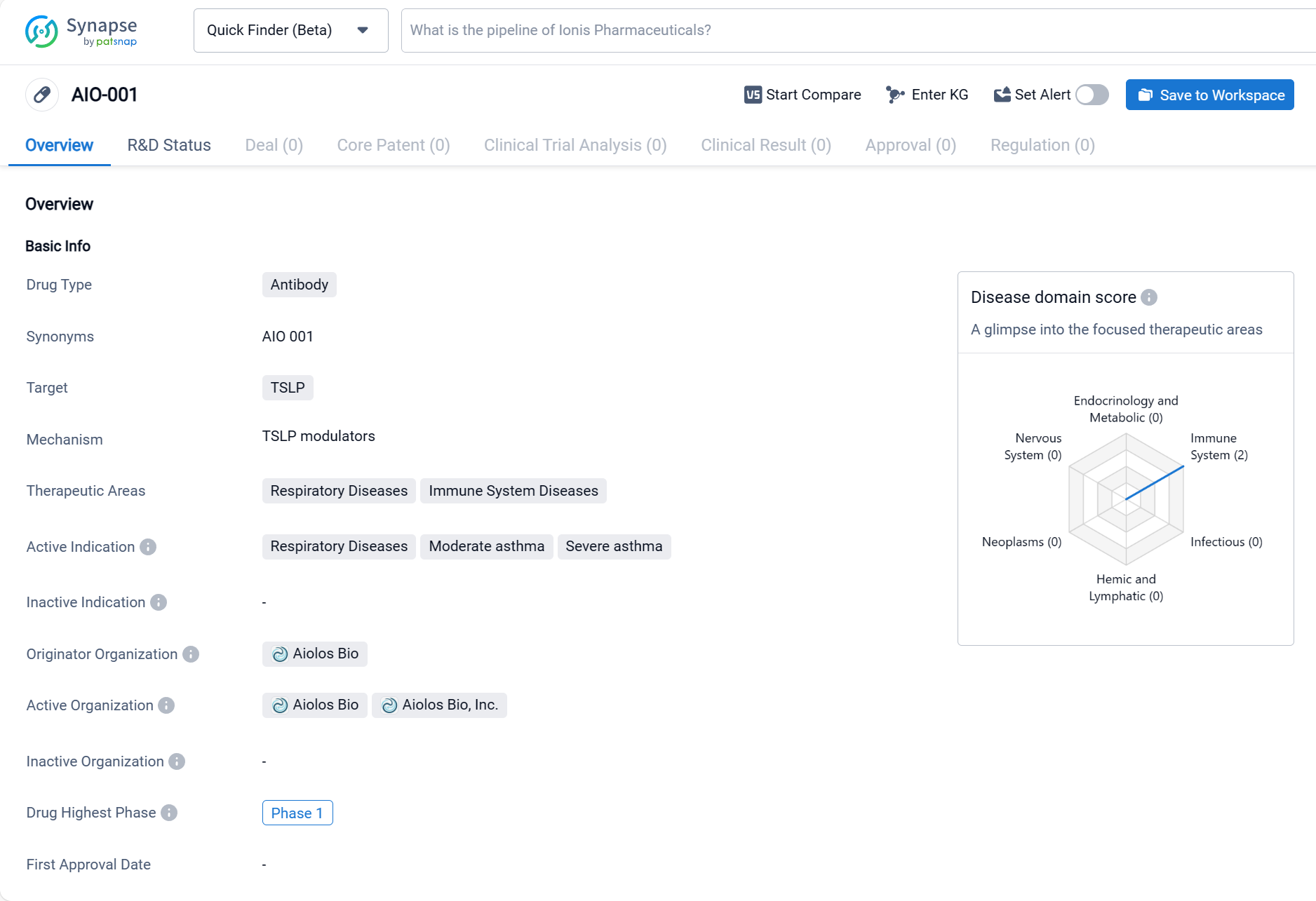
AIO-001 is an antibody drug developed by Aiolos Bio, targeting TSLP (Thymic Stromal Lymphopoietin). It falls under the category of biomedicine and is primarily intended for the treatment of respiratory diseases and immune system diseases. The drug's active indications include respiratory diseases, moderate asthma, and severe asthma. As of the latest available information, AIO-001 has reached the highest phase of development, which is Phase 1. Click the image below to directly embark on the exploration journey with the AIO-001!
In the clinical research, the drug AIO-001 has demonstrated good safety, tolerability, pharmacokinetics, and biological activity in both healthy volunteers and asthma patients, with low immunogenicity. The drug received approval from the National Medical Products Administration in May 2021 to conduct clinical trials for the treatment of asthma, and in May this year, it was also approved to conduct clinical trials for the treatment of chronic rhinosinusitis with nasal polyps. Currently, both studies are in Phase 2 clinical trials.
About Aiolos Bio
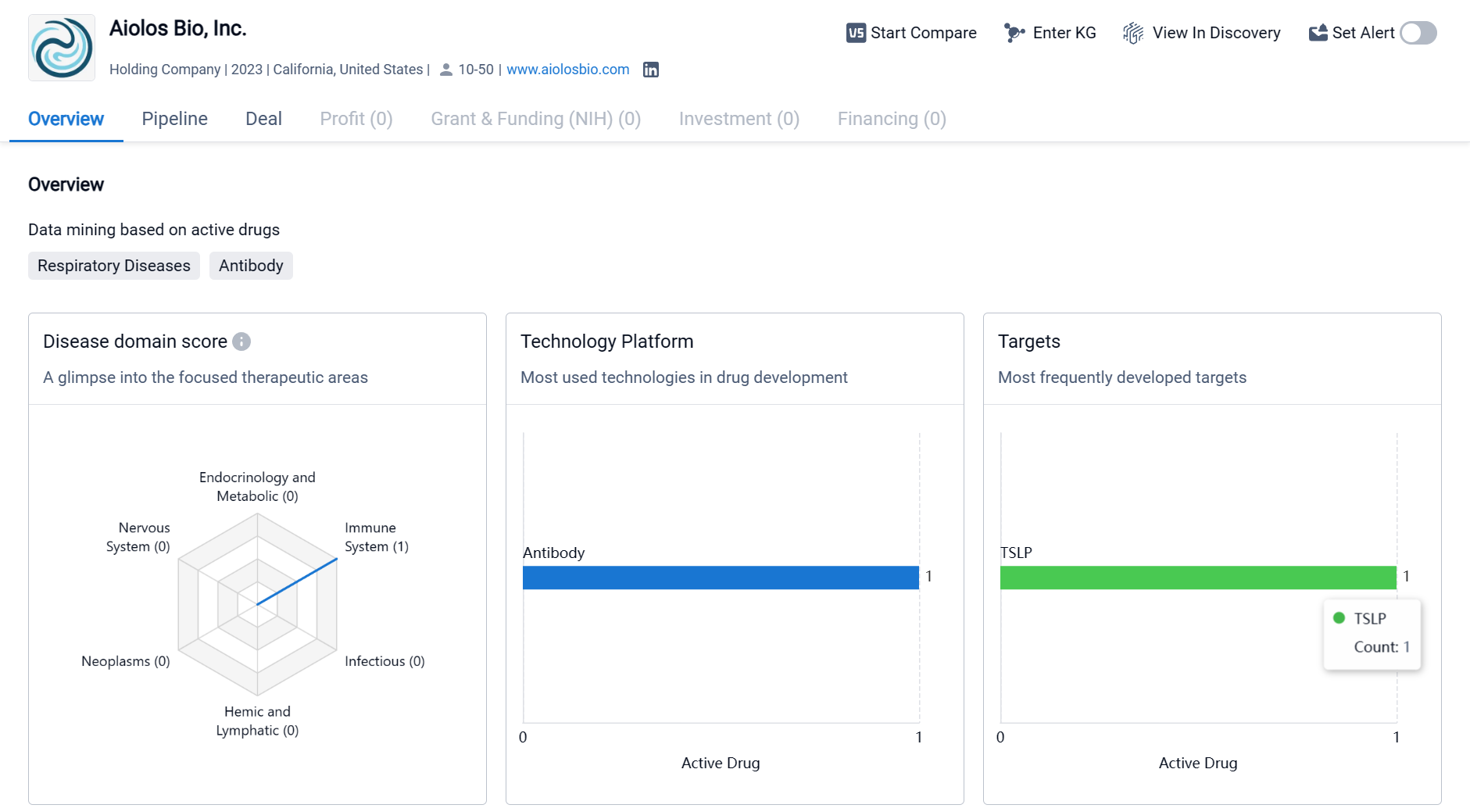
Aiolos Bio, Inc. is a biomedicine organization founded in 2023 and based in California, United States. The company focuses on developing drugs for immune system diseases and respiratory diseases, with a particular emphasis on targeting the TSLP cytokine. While the company has made progress in its drug development efforts, it is still in the early stages of its pipeline, with one drug in Phase 1 of development. As a business development expert, it is important to closely monitor the progress of Aiolos Bio, Inc. and assess its potential in the competitive pharmaceutical industry.
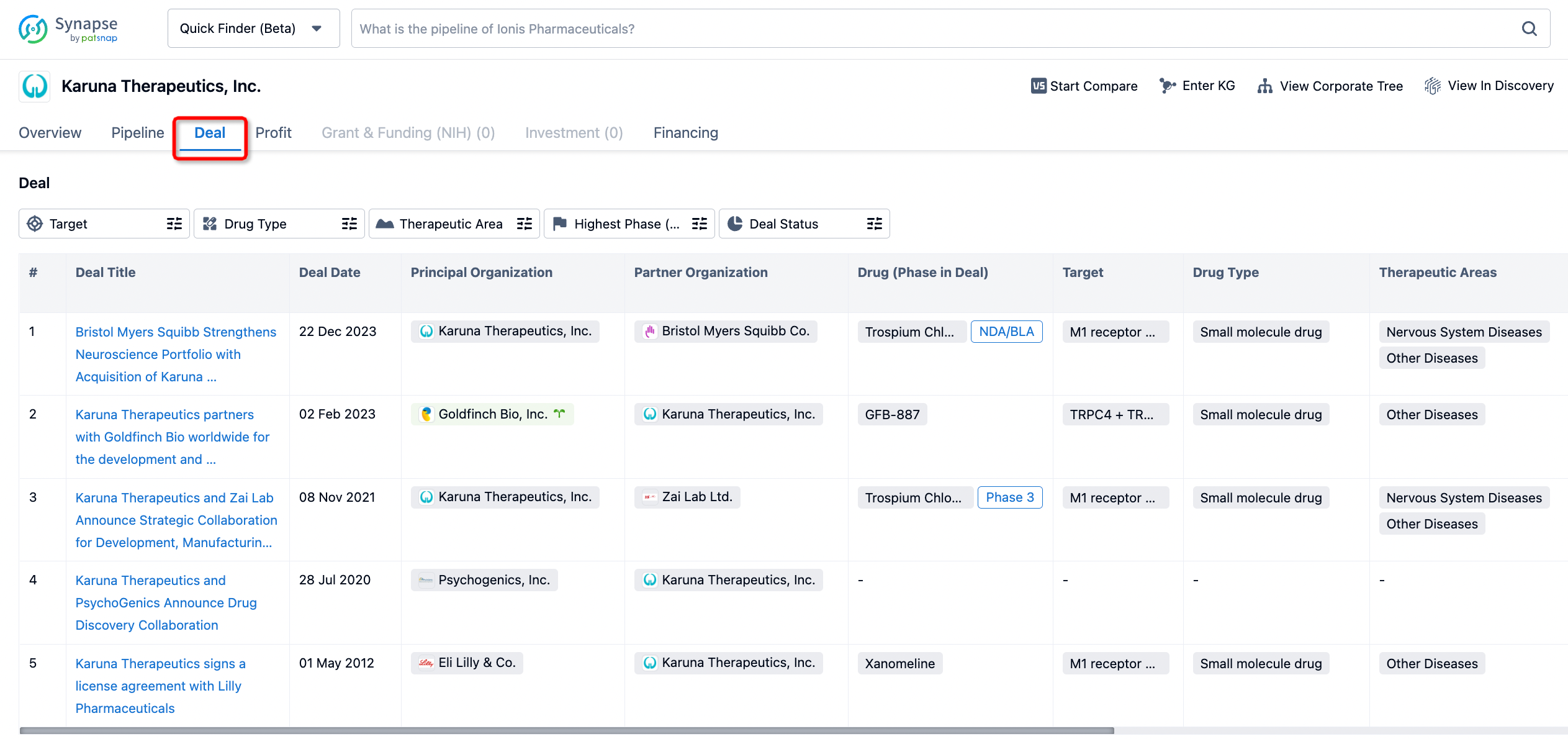
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
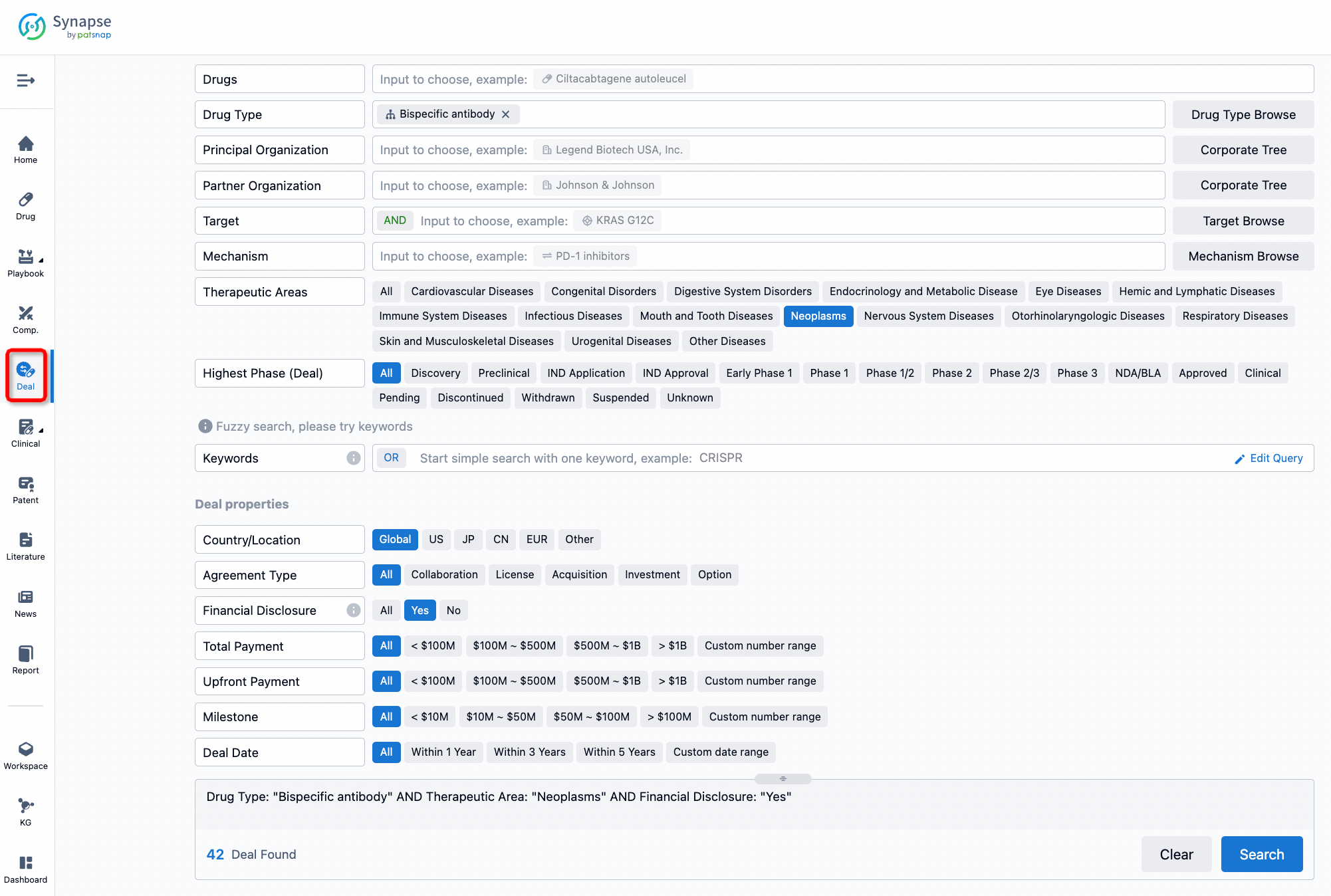
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
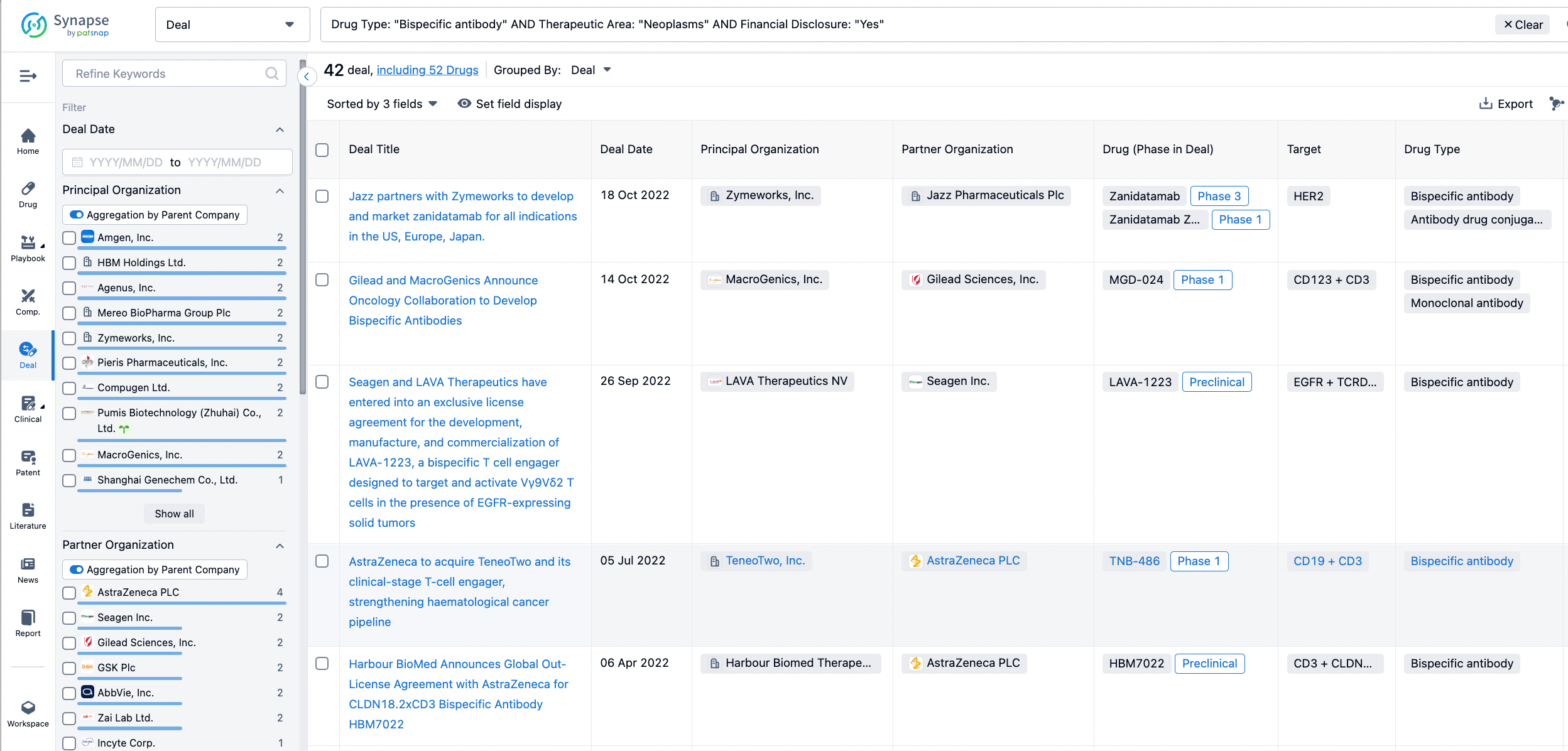
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
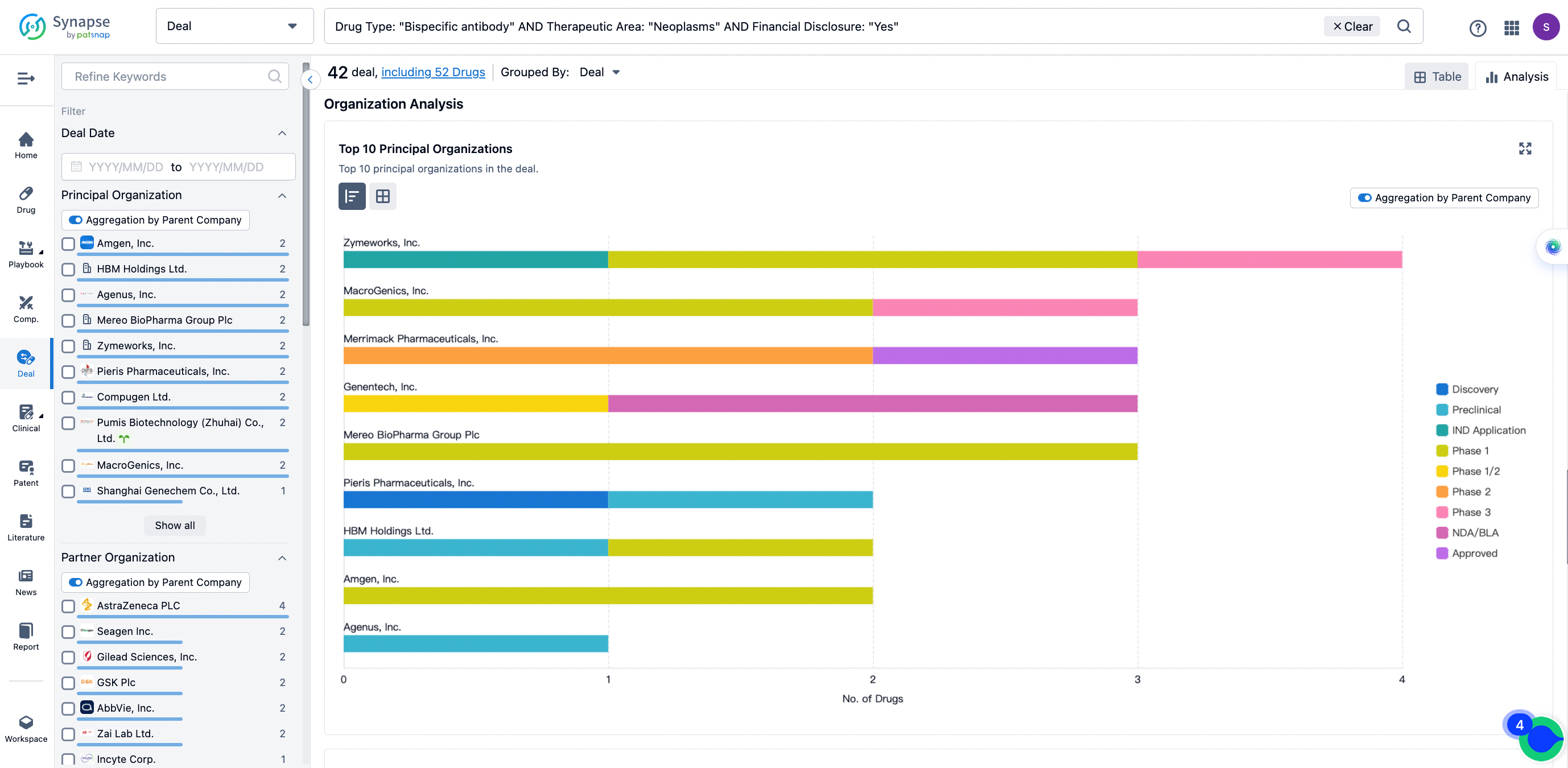
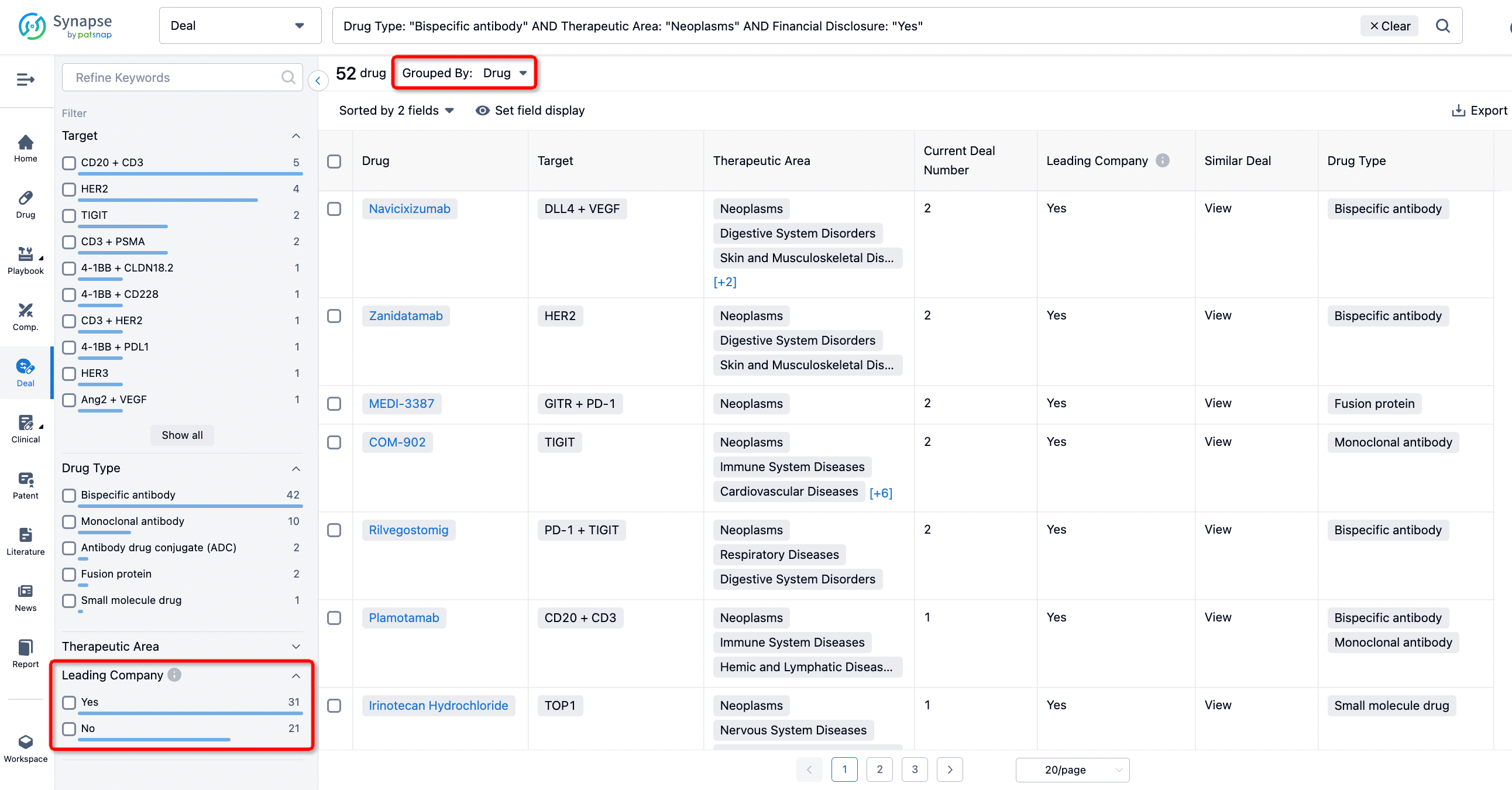
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
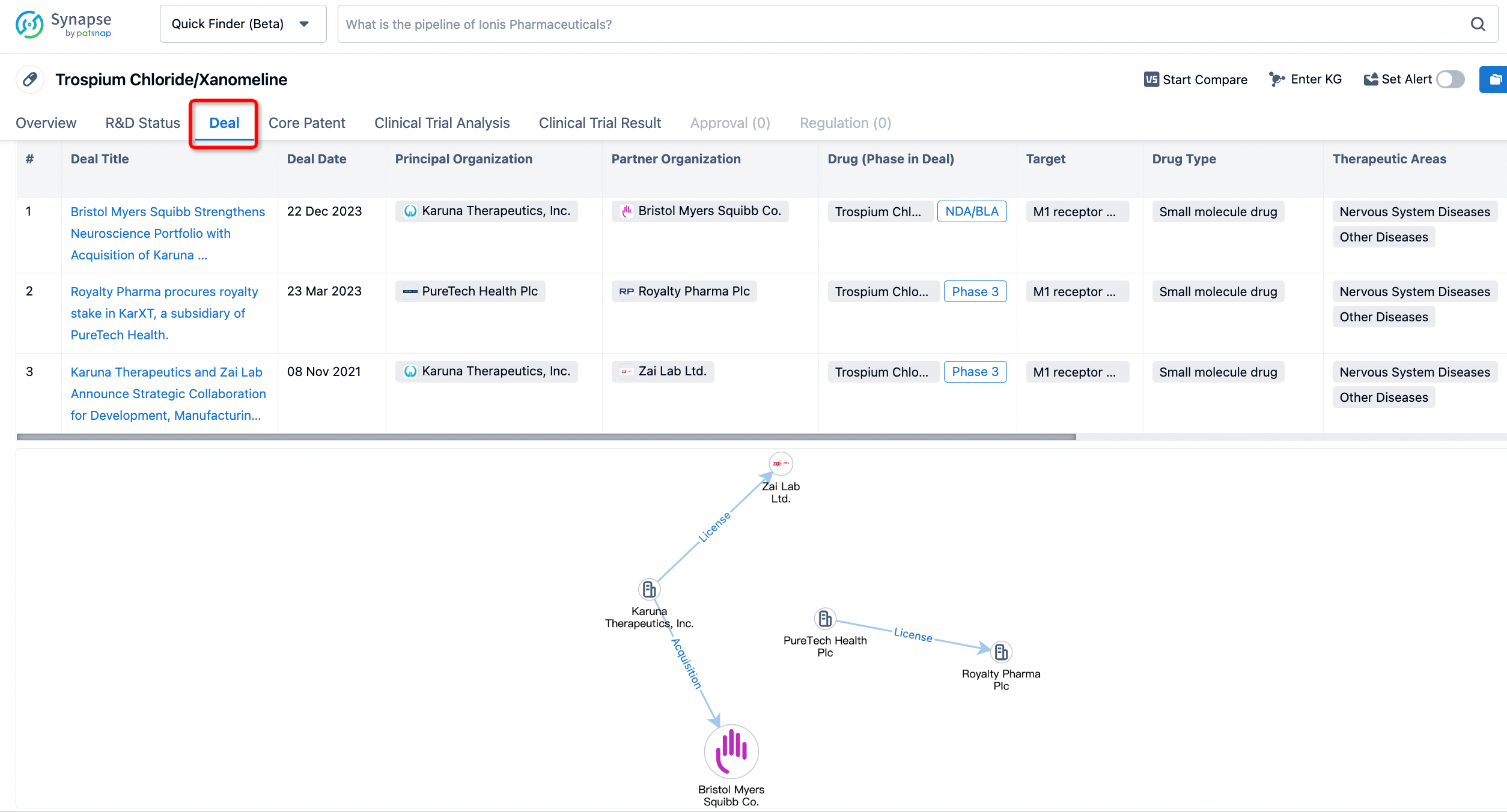
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
Click on the image below to embark on a brand new journey of drug discovery!