Xeris Biopharma Secures Global Exclusive Rights to Distribute Xeriject® Version of Teprotumumab
Xeris Biopharma Holdings, Inc. has disclosed the formation of a unique global licensing deal granting Amgen the rights to advance, produce, and market a subcutaneous version of teprotumumab. This will incorporate the utilization of Xeris’ proprietary XeriJect® system specifically for Thyroid Eye Disease, which is characterized as a severe, advancing, and rare autoimmune disorder that may pose a threat to eyesight. Within the United States, the drug Teprotumumab-trbw is commercially recognized under the brand name TEPEZZA®.
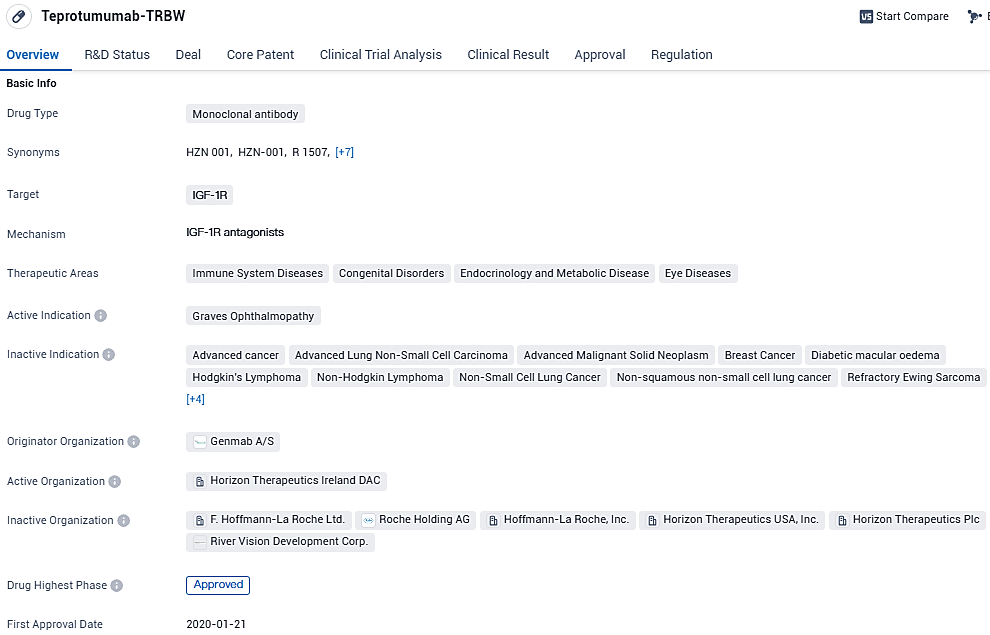
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In affirming the licensing of XeriJect, Paul R. Edick, the CEO and Chairman of Xeris, expressed confidence in their innovation's capacity to facilitate the administration of large molecule drugs through subcutaneous injections. Edick highlighted the potential for their technology to result in treatments that are more efficient, safe, and user-friendly, possibly increasing patient compliance with the treatment process. He also expressed Xeris' commitment to rapidly advancing this pivotal venture with their collaborator.
As specified in the licensing contract, Xeris stands to gain up to $75 million from various developmental, regulatory achievements, and future sales benchmarks. Additionally, the company is eligible for cumulative royalties in the single-digit percentage range from the sales of TEPEZZA, which incorporates the XeriJect system.
The XeriJect platform features cutting-edge, ready-to-utilize, viscoelastic suspension formulations designed to enhance how medicines are dispensed. These advances aim to reduce the complexity of treatments and to have a positive impact on patient health across a variety of medical specializations.
Designed to hold drug concentrations exceeding 400mg/mL, XeriJect suspensions are optimized for low-volume subcutaneous delivery while maintaining stability without sedimentation during storage. The formulations employ excipients sanctioned by the FDA and capitalize on established production techniques. XeriJect's formulation technology is highly adaptable for use with a variety of drugs and biological products, including large molecules like proteins, monoclonal antibodies, and vaccines. The technology is safeguarded by a comprehensive portfolio of patents, trade secrets, and proprietary expertise, and it is currently on offer for licensing partnerships.
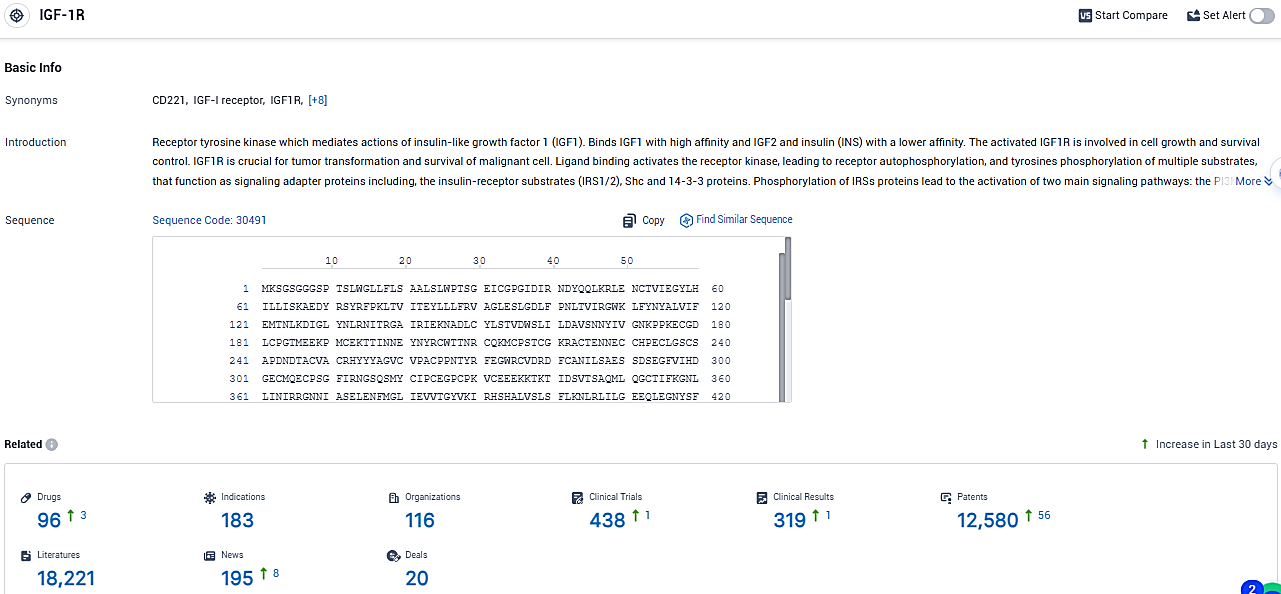
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 15, 2024, there are 96 investigational drugs for the IGF-1R target, including 183 indications, 116 R&D institutions involved, with related clinical trials reaching 438, and as many as 12580 patents.
Teprotumumab-TRBW is a monoclonal antibody drug that targets the IGF-1R. It has received approval for the treatment of Graves Ophthalmopathy and falls under various therapeutic areas. The drug has reached the highest phase of development and has been granted regulatory designations such as Fast Track, Orphan Drug, and Breakthrough Therapy. Its approval in the United States in January 2020 marks an important milestone in the field of biomedicine.