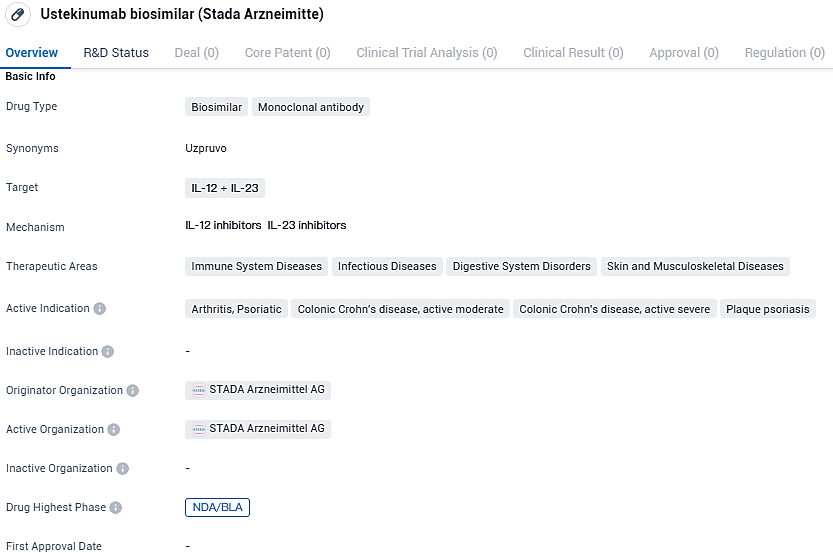
STADA and Alvotech obtain green light for Uzpruvo, the inaugural European biosimilar to Stelara, based on ustekinumab
The European Commission has granted STADA and Alvotech approval to market Uzpruvo® (AVT04), which is a biosimilar corresponding to the original biologic, Stelara® (ustekinumab). This marks the inaugural approval for a ustekinumab biosimilar across the entire European Economic Area (EEA), encompassing the 27 nations of the European Union along with the nations of Iceland, Liechtenstein, and Norway.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The granting of the European marketing authorization for Uzpruvo clears a path for its entry into the market immediately after the SPC for Stelara expires in July 2024. With a market worth €2.5 billion in the EU for ustekinumab, the introduction of biosimilar versions could drastically improve the availability of this transformative biologic treatment across the disciplines of gastroenterology, dermatology, and rheumatology.
Bryan Kim, who heads the Global Specialty division at STADA, stated: "Europe's first approval of a ustekinumab biosimilar has the capability to greatly enhance access for patients through increased market competition. STADA is eager to supplement its existing suite of six commercialized biosimilars by providing an additional, cost-efficient therapeutic alternative to professionals in gastroenterology, dermatology, and rheumatology."
Alvotech's Chief Commercial Officer, Anil Okay, expressed enthusiasm: "Alvotech is poised to lead the charge in introducing biosimilar competition within the ustekinumab segment, just as we have previously achieved with our adalimumab biosimilar last year, aiming to elevate access to biologic treatments for inflammatory conditions among patients."
Ustekinumab is classified as a human IgG1κ monoclonal antibody. Uzpruvo, manufactured in Sp2/0 cells and employing a perfusion method akin to the original product Stelara, selectively binds to the p40 subunit shared by IL-12 and IL-23 cytokines, which are vital in addressing immune-mediated conditions such as Crohn’s disease, psoriasis, and psoriatic arthritis.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
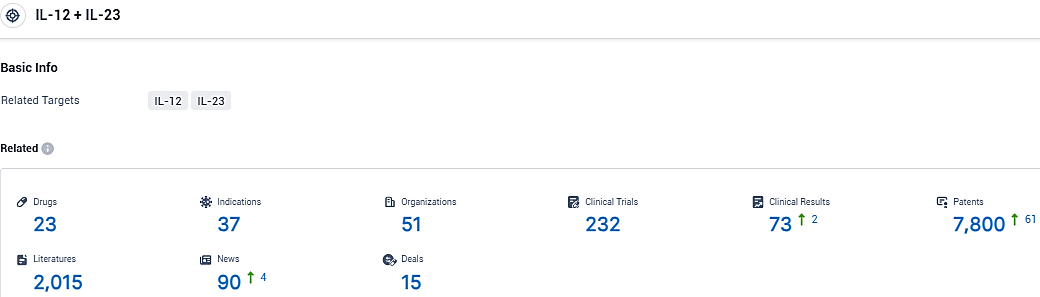
According to the data provided by the Synapse Database, As of January 15, 2024, there are 23 investigational drugs for the IL-12 and IL-23 target, including 37 indications, 51 R&D institutions involved, with related clinical trials reaching 232, and as many as 7800 patents.
The European Commission’s decision to issue a marketing authorization came after the Committee for Medicinal Products for Human Use within the European Medicines Agency’s in November 2023 adopted a positive opinion on approving Uzpruvo with the indications Crohn’s disease, psoriasis and psoriatic arthritis.