Exploring Valrubicin's Revolutionary R&D Successes and its Mechanism of Action
Valrubicin 's R&D Progress
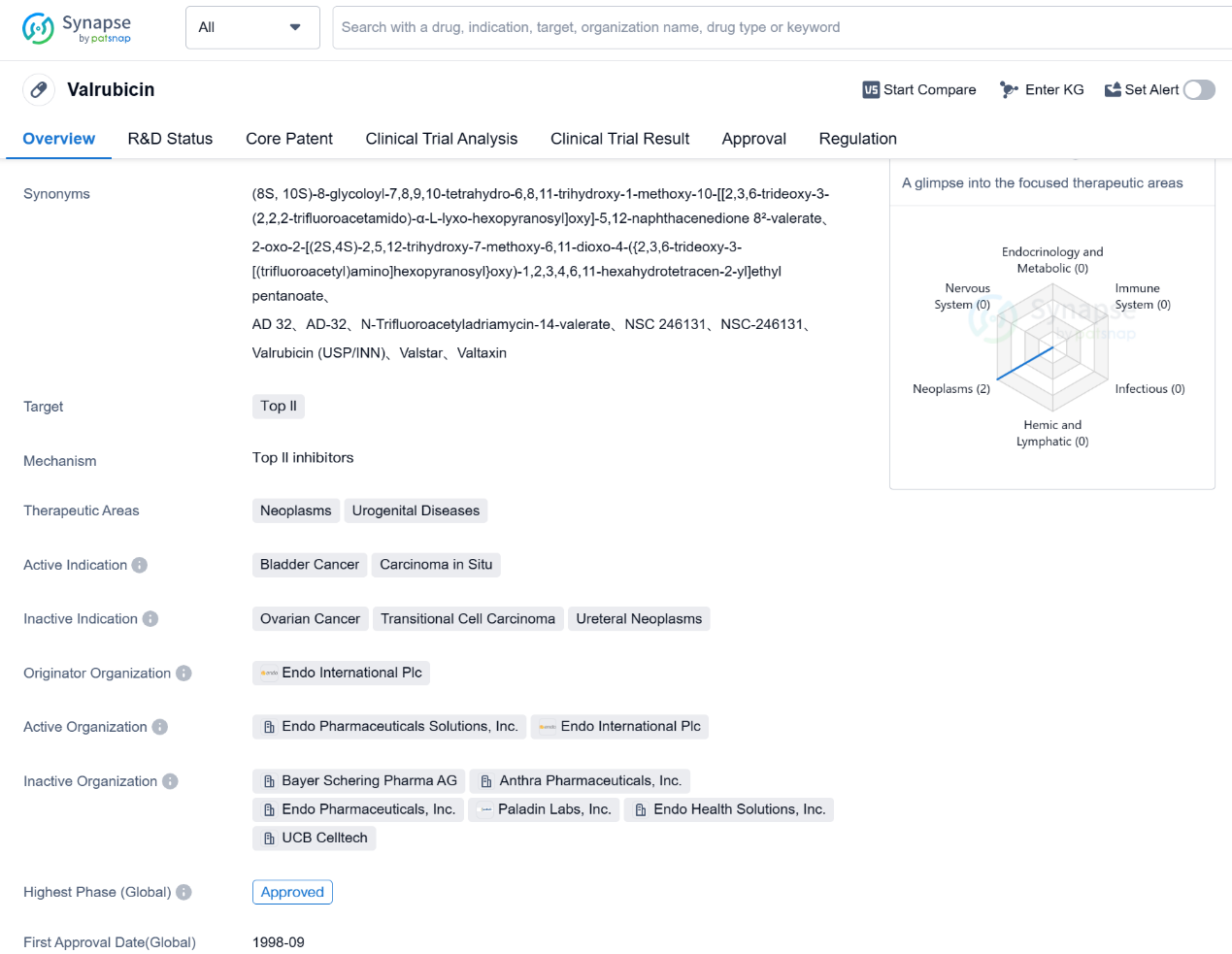
Valrubicin is a small molecule drug that falls under the therapeutic areas of neoplasms and urogenital diseases. It specifically targets Top II, which is an enzyme involved in DNA replication and repair. The drug is primarily used for the treatment of bladder cancer and carcinoma in situ.
Valrubicin was first approved in the United States in September 1998, making it an established drug in the market. It is important to note that the drug has received orphan drug designation, which indicates that it is intended to treat rare diseases or conditions. This regulatory status may provide certain benefits to the drug's manufacturer, such as market exclusivity and financial incentives.
The originator organization of Valrubicin is Endo International Plc, a pharmaceutical company that specializes in developing and commercializing innovative therapies. As the highest phase of development for Valrubicin is approved, it suggests that the drug has successfully completed clinical trials and has been deemed safe and effective for its intended use.
Bladder cancer is a type of cancer that affects the tissues of the bladder, and carcinoma in situ refers to cancer that is confined to the surface layer of the bladder. Valrubicin is specifically indicated for the treatment of these conditions, indicating its potential efficacy in managing bladder cancer and carcinoma in situ.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Valrubicin: Top Ⅱ inhibitor
Top II inhibitor is a term that can be explained from a biomedical perspective. Top II refers to topoisomerase II, which is an enzyme involved in DNA replication and repair. It plays a crucial role in untangling and unwinding DNA during these processes. Top II inhibitors are a class of drugs that specifically target and inhibit the activity of topoisomerase II.
By inhibiting the action of topoisomerase II, these drugs prevent the enzyme from properly functioning, leading to the accumulation of DNA breaks and preventing DNA from being properly replicated and repaired. This ultimately disrupts the growth and division of cancer cells, as well as other rapidly dividing cells.
Top II inhibitors are commonly used in cancer treatment, as they can effectively target and kill cancer cells. Examples of top II inhibitors include etoposide, doxorubicin, and mitoxantrone. These drugs are often used in combination with other chemotherapy agents to enhance their effectiveness.
It is important to note that top II inhibitors can have side effects, as they can also affect normal, healthy cells that undergo rapid division, such as cells in the bone marrow and gastrointestinal tract. Therefore, their use requires careful monitoring and management by healthcare professionals.
Drug Target R&D Trends for Valrubicin
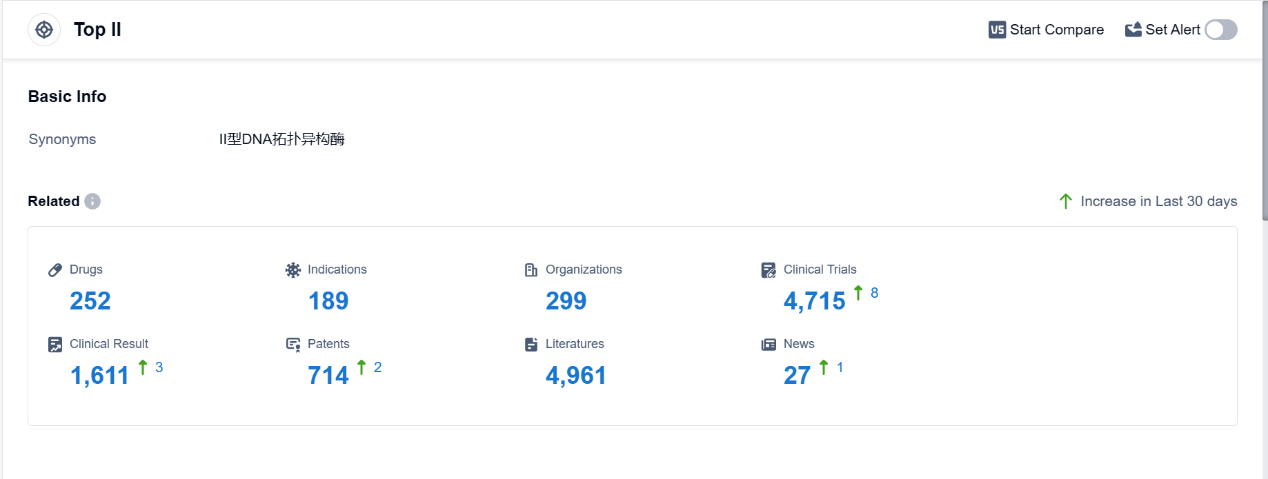
Top II, also known as topoisomerase II, is an essential enzyme found in the human body that plays a crucial role in DNA replication and repair. It is responsible for managing the topological changes in DNA structure by introducing temporary breaks in the DNA strands. This allows for the unwinding and separation of DNA during replication and transcription processes. Top II is involved in regulating the supercoiling of DNA, ensuring its proper functioning and stability. Additionally, this enzyme is a target for certain pharmaceutical drugs, as inhibiting its activity can disrupt DNA replication and lead to cell death, making it an important target for cancer treatments. According to PatSnap Synapse, as of 4 Sep 2023, there are a total of 252 Top II drugs worldwide, from 299 organizations, covering 189 indications, and conducting 4715 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Valrubicin is a small molecule drug that targets Top II and is primarily used for the treatment of bladder cancer and carcinoma in situ. It was first approved in the United States in 1998 and has received orphan drug designation. As an approved drug, Valrubicin has undergone rigorous testing and has been deemed safe and effective for its intended use. Its originator organization is Endo International Plc, a pharmaceutical company specializing in innovative therapies.