Decoding Naxitamab: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Naxitamab's R&D Progress
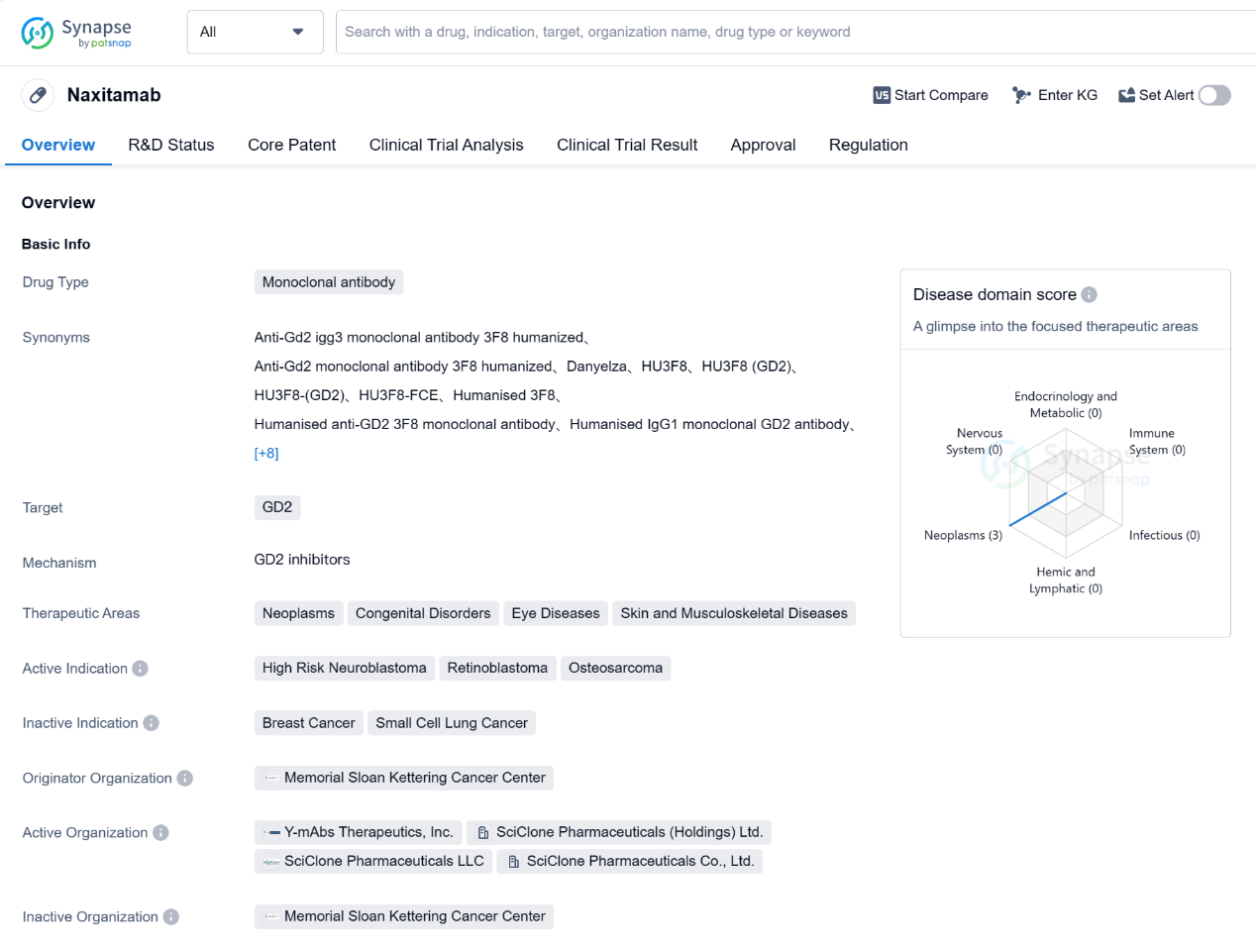
Naxitamab is a monoclonal antibody drug that targets GD2, a protein found on the surface of certain cancer cells. It is primarily used in the treatment of high-risk neuroblastoma, retinoblastoma, and osteosarcoma. These therapeutic areas include neoplasms (abnormal growth of cells), congenital disorders, eye diseases, and skin and musculoskeletal diseases.
The drug was developed by Memorial Sloan Kettering Cancer Center, a renowned organization in the field of cancer research and treatment. Naxitamab has received approval in both the United States and China, indicating its effectiveness and safety in treating the approved indications.
In terms of regulatory status, Naxitamab has undergone priority review, accelerated approval, and has been designated as an orphan drug and breakthrough therapy. Priority review and accelerated approval processes expedite the evaluation and approval of drugs that address unmet medical needs. Orphan drug designation is granted to drugs that treat rare diseases, while breakthrough therapy designation is given to drugs that show significant improvement over existing treatments for serious conditions.
The highest phase of development for Naxitamab is approved, indicating that it has successfully completed clinical trials and demonstrated its efficacy and safety. The first approval of Naxitamab took place in November 2020 in the United States.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Naxitamab: GD2 Inhibitors
GD2 inhibitors are a type of drugs that specifically target and inhibit the activity of GD2, which is a glycolipid molecule found on the surface of certain cancer cells. From a biomedical perspective, GD2 inhibitors are used in the field of oncology to treat neuroblastoma, a type of cancer that commonly affects children. Neuroblastoma cells often express high levels of GD2, making it a suitable target for therapeutic intervention. GD2 inhibitors work by binding to GD2 on the cancer cells, which can lead to the activation of immune cells to attack and destroy the tumor cells. This approach is known as immunotherapy and has shown promising results in improving the outcomes for patients with neuroblastoma.
Drug Target R&D Trends for Naxitamab
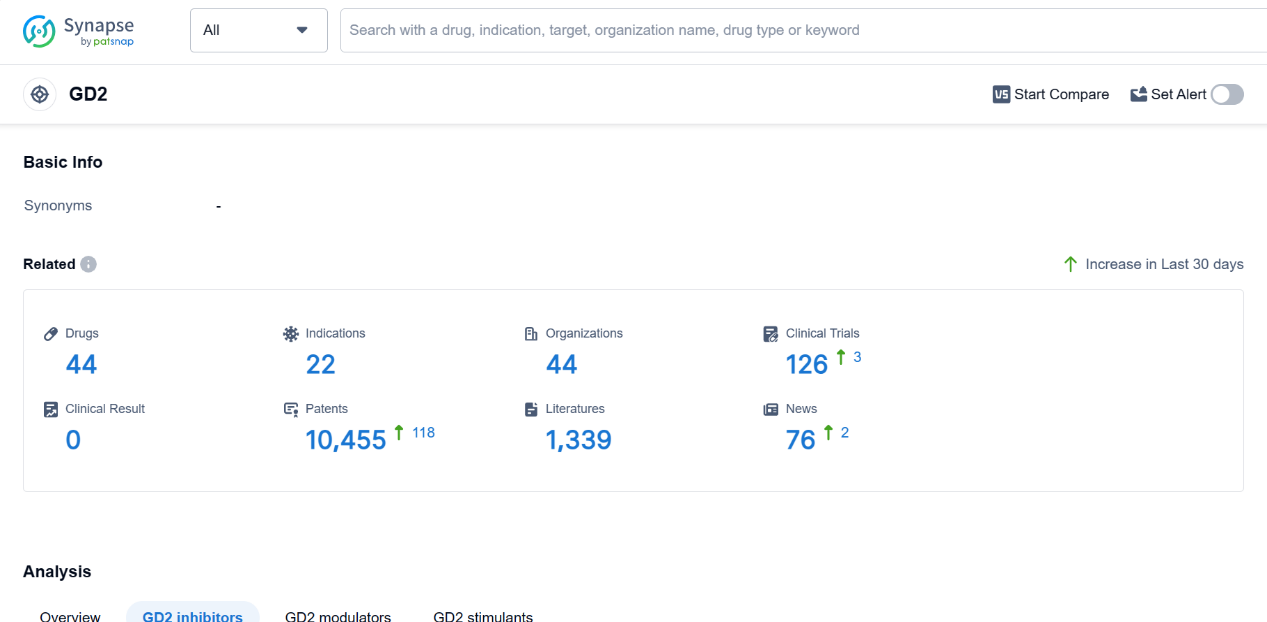
A GD2 is a glycolipid molecule found on the surface of cells in the human body. It plays a crucial role in cell adhesion and signaling processes. GD2 is particularly abundant in the nervous system, where it is involved in the development and maintenance of neuronal connections. Additionally, GD2 has been identified as a marker for certain types of cancer cells, including neuroblastoma and melanoma. This makes it an attractive target for immunotherapy and targeted drug delivery strategies in the pharmaceutical industry. Understanding the role of GD2 in the human body is essential for developing effective treatments for diseases associated with its expression.
According to PatSnap Synapse, as of 4 Sep 2023, there are a total of 44 GD2 drugs worldwide, from 44 organizations, covering 22 indications, and conducting 126 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Naxitamab is a small molecule drug developed by Pfizer Inc. It targets PPARα and is indicated for the treatment of hyperlipidemias and hypertriglyceridemia. With approvals in the global market, Naxitamab has a long-standing presence in the pharmaceutical industry since its first approval in the United States in 1967.