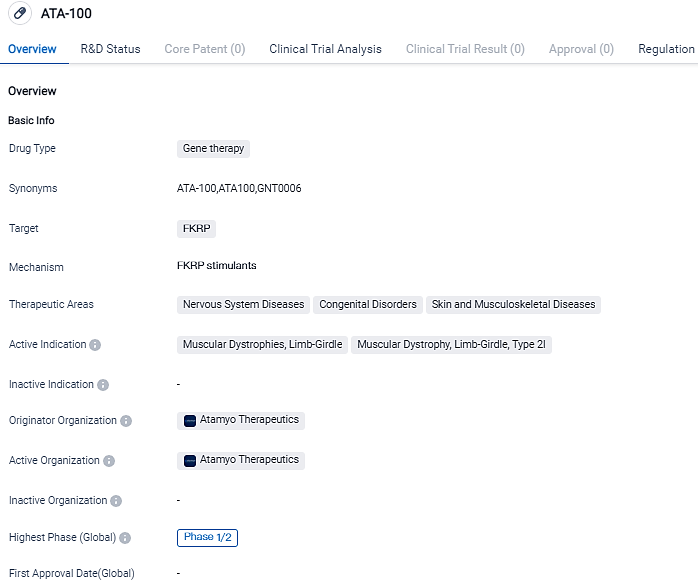
FDA authorizes initiation of IND for ATA-100, a Genetic Treatment for Limb-Girdle Muscular Dystrophy Type 2I/R9 (LGMD2I/R9)
Atamyo Therapeutics, which is committed to advancing new gene therapies specifically for muscular dystrophies and cardiomyopathies, proclaimed that the FDA has given the green light for its ATA-100 IND application to advance into a Phase 1b/2b clinical study. ATA-100 is a unique gene therapy proposed for the remediation of FKRP limb-girdle muscular dystrophy Type 2I/R9 (LGMD2I/R9).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
ATA-100, previously identified as GNT0006, is currently undergoing assessment across multiple medical facilities in a phase 1b/2b trial taking place within Denmark, France, and the United Kingdom.
Dr. Sophie Olivier, the Chief Medical Officer at Atamyo, declared that receiving approval for an Investigational New Drug (IND) is a decisive advancement in the process of introducing ATA-100 to American patients battling the acutely crippling disease, LGMD-R9. "Having started a phase 1b/2b trial in Europe in 2022, we are set to commence clinical trials at U.S. sites in the foreseeable future," she stated.
The rare genetic condition, LGMD2I/R9, is triggered by alterations in the gene responsible for producing FKRP. Around 5,000 individuals in the U.S and Europe are affected by this disease. The condition typically manifests during late childhood or early adulthood, leading to progressive muscular deterioration resulting in the inability to walk. Patients showing an increased vulnerability to respiratory impairment. Sadly, there are no existing remedial treatments for LGMD2I/R9 at present.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
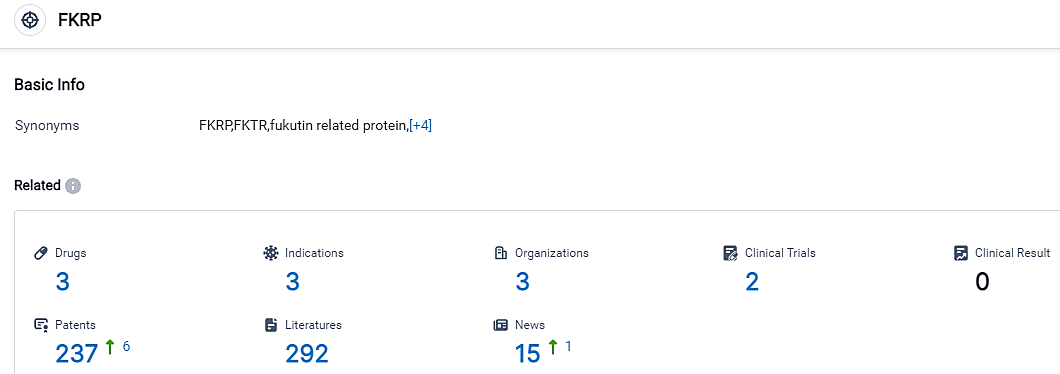
According to the data provided by the Synapse Database, As of September 8, 2023, there are 3 drugs for the FKRP target, including 3 indications, with related clinical trials reaching 2,and as many as 237 patents.
FKRP is distinctive for having several firms actively engaged in pharmaceutical development. The frontrunner in terms of medication quantity and R&D progress is Atamyo Therapeutics, with medications in Phase 1/2 and IND Application phases. Muscular Dystrophies, Limb-Girdle, and Muscular Dystrophy, Limb-Girdle, Type 2I are the conditions with the most drugs in progress. In general, FKRP has a lot of potential for development, especially in the fields of gene therapy and muscular dystrophies.