FDA Approves BlueRock Therapeutics' IND for iPSC-Derived Cell Therapy OpCT-001 Targeting Photoreceptor Diseases
BlueRock Therapeutics LP, a clinical-stage cell therapy company and fully owned, independently managed subsidiary of Bayer AG, has received approval from the U.S. Food and Drug Administration (FDA) for its Investigational New Drug (IND) application for OpCT-001. This investigational therapy, which is derived from induced pluripotent stem cells (iPSCs), targets primary photoreceptor diseases.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are gratified by the significant advancements we have achieved so far and are excited that the FDA has given the green light for our IND application to begin clinical trials for OpCT-001," stated Amit Rakhit, Chief Development and Medical Officer at BlueRock Therapeutics. "We believe that OpCT-001 has the potential to restore vision in individuals with primary photoreceptor diseases and are eager to collaborate with the ophthalmic community as we launch our Phase 1/2a clinical trial."
OpCT-001 represents the first investigational therapy derived from iPSCs to be clinically assessed for primary photoreceptor diseases. Activities to kickstart a Phase 1/2a trial are already in motion. This study, a first-in-human trial, is structured to evaluate the safety and tolerability of subretinal delivery of OpCT-001 in patients with primary photoreceptor diseases, in addition to assessing its impact on retinal architecture, visual function, and functional vision. The trial will examine multiple dose levels of OpCT-001 and plans to recruit participants from various U.S. sites.
Primary photoreceptor diseases form a subset of inherited retinal disorders encompassing retinitis pigmentosa and cone-rod dystrophy. These conditions impair the structure and function of photoreceptor cells in the retina, resulting in irreversible vision loss in both children and adults. Around 110,000 individuals in the U.S. are affected by these diseases, and treatment options remain limited. OpCT-001 seeks to address vision loss by substituting degenerated retinal cells with functional ones.
In January 2024, OpCT-001 was exclusively licensed from FUJIFILM Cellular Dynamics and Opsis Therapeutics, as part of a strategic R&D and clinical manufacturing partnership between BlueRock Therapeutics, FUJIFILM Cellular Dynamics, and Opsis Therapeutics, which began in 2021. Through this collaboration, FUJIFILM Cellular Dynamics has supported BlueRock Therapeutics in research and development, along with executing key IND-enabling activities, including the clinical manufacturing of OpCT-001 at their cGMP² facility in Madison, Wisconsin.
OpCT-001 is an investigational therapy that has not received approval from any regulatory body, and its efficacy and safety remain to be conclusively established.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
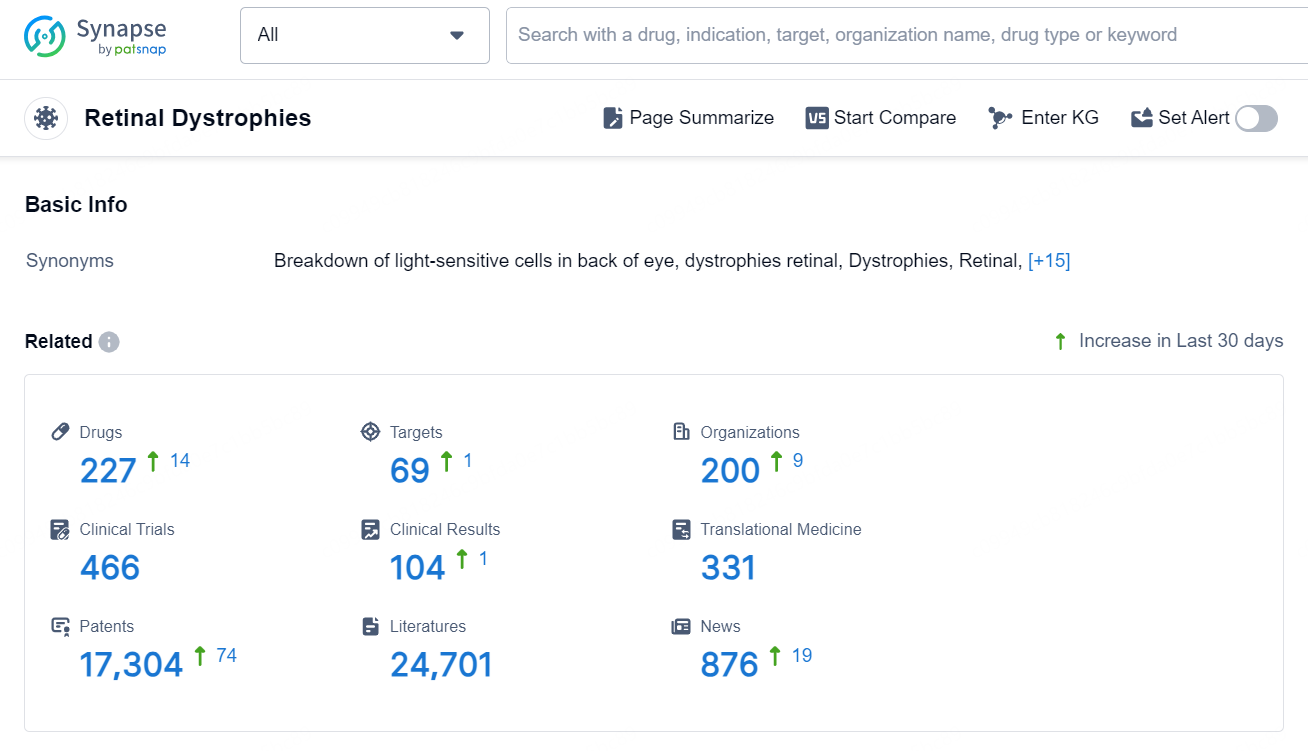
According to the data provided by the Synapse Database, As of September 5, 2024, there are 227 investigational drugs for the Retinal Dystrophies, including 69 targets, 200 R&D institutions involved, with related clinical trials reaching 466, and as many as 17304 patents.
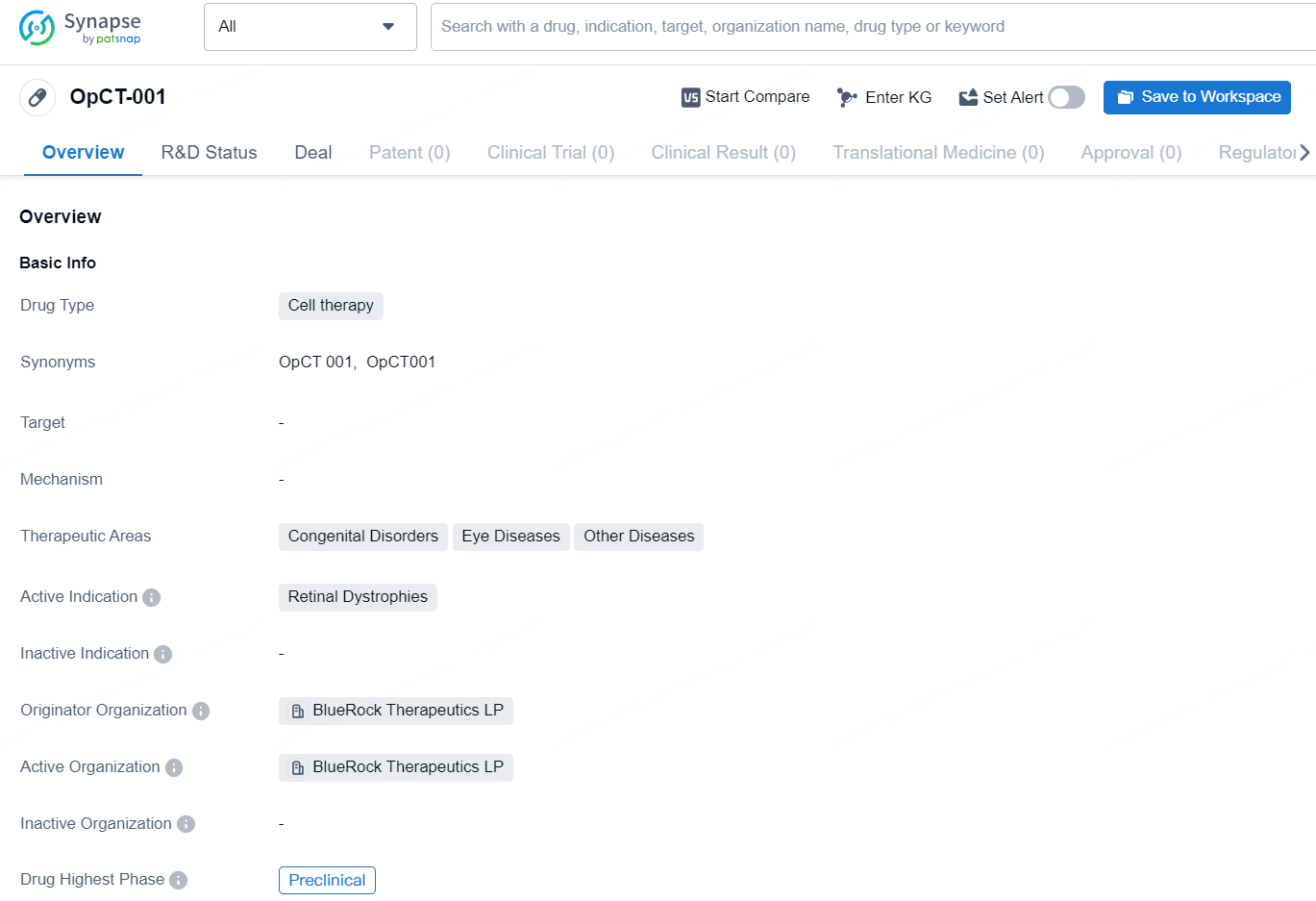
OpCT-001 is a cell therapy drug being developed by BlueRock Therapeutics LP. It falls under the therapeutic areas of Congenital Disorders, Eye Diseases, and other diseases, with the active indication being Retinal Dystrophies. The drug is currently in the preclinical phase, indicating that it is still undergoing testing and has not yet progressed to clinical trials.