FDA Approves Breyanzi for Relapsed/Refractory Follicular Lymphoma
Bristol Myers Squibb revealed that the U.S. Food and Drug Administration has given fast-track approval to Breyanzi (lisocabtagene maraleucel; liso-cel), which is a CD19-directed chimeric antigen receptor T cell therapy. This therapy is approved for treating adult patients with follicular lymphoma that has either relapsed or not responded to at least two previous systemic treatments.
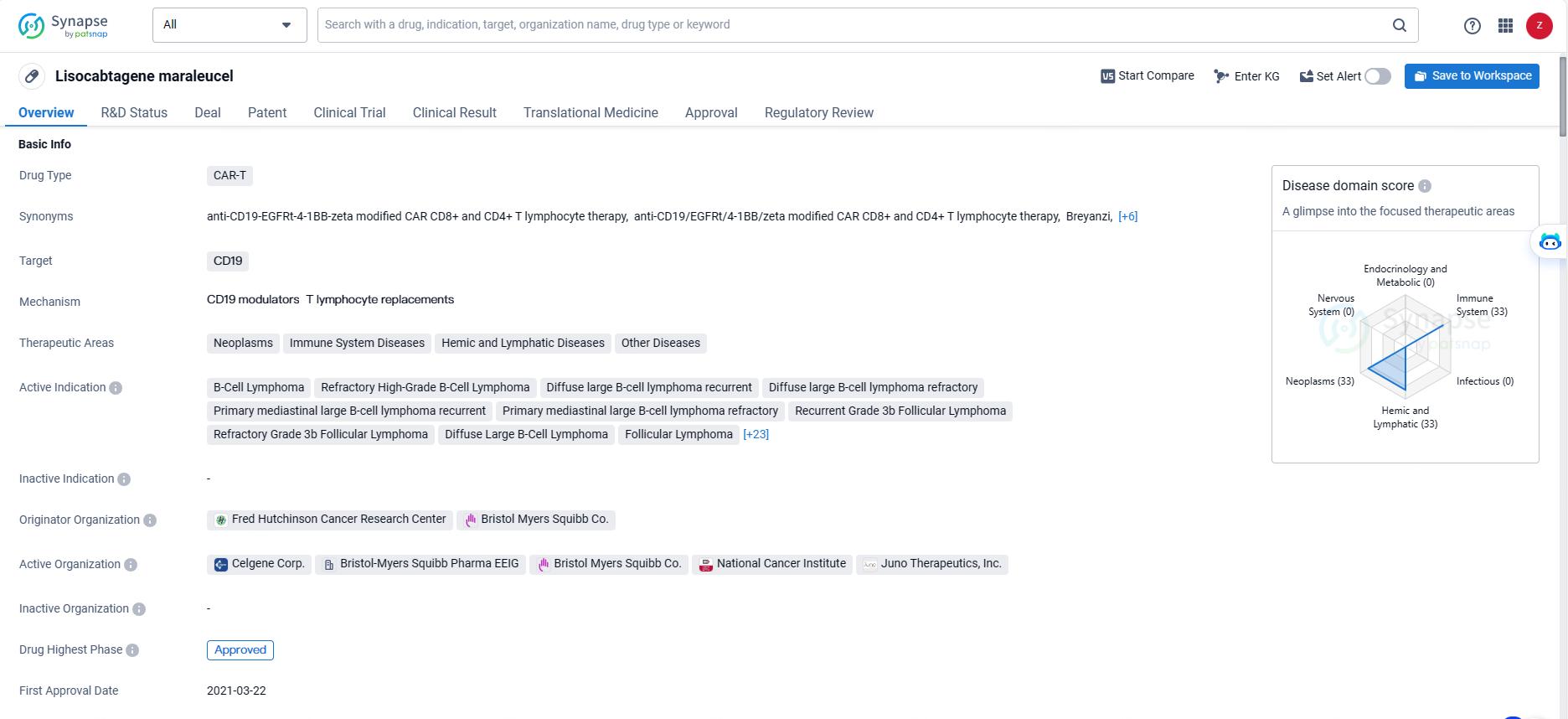
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
This indication has received accelerated approval based on the response rate and the duration of the response. Further approval for this indication may depend on confirming and describing clinical benefits in a confirmatory trial.
Breyanzi is now part of the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for B-cell Lymphomas, receiving a Category 2A recommendation for third-line and subsequent treatments for relapsed or refractory FL.
For relapsed or refractory FL, Breyanzi is administered as a single infusion** with a dose containing 90 to 110 x 10^6 CAR-positive viable T cells. Refer to the Important Safety Information section below, including Boxed WARNINGS regarding Cytokine Release Syndrome, Neurologic Toxicities, and Secondary Hematological Malignancies for Breyanzi.
“Breyanzi is a key element of our cell therapy portfolio, offering a unique profile across various B-cell malignancies,” stated Bryan Campbell, senior vice president and Head of Commercial, Cell Therapy, Bristol Myers Squibb. “Today’s approval of Breyanzi for relapsed or refractory FL offers an option that has the potential for sustained remission in a single infusion and a safety profile that supports its administration and monitoring in both inpatient and outpatient settings in an increasing number of certified treatment centers across the U.S.”
Historically, FL has been seen as an incurable condition, with patients commonly experiencing relapses after front-line therapy, and their prognosis worsens with each subsequent relapse. Despite the advancements in treatment, there is still a significant need for additional options that provide treatment-free intervals with durable, complete responses.
“In treating relapsed or refractory follicular lymphoma, patients often go through multiple treatments with typically shorter response durations in subsequent therapies. Those who experience early disease progression tend to have a particularly poor prognosis,” said M. Lia Palomba, M.D., TRANSCEND investigator, and lymphoma and cell therapy specialist at Memorial Sloan Kettering Cancer Center. “The FDA’s approval of liso-cel for relapsed or refractory FL patients represents a significant development in addressing an existing unmet need in the FL treatment landscape, offering a new option with notably high response rates and an established safety profile.”
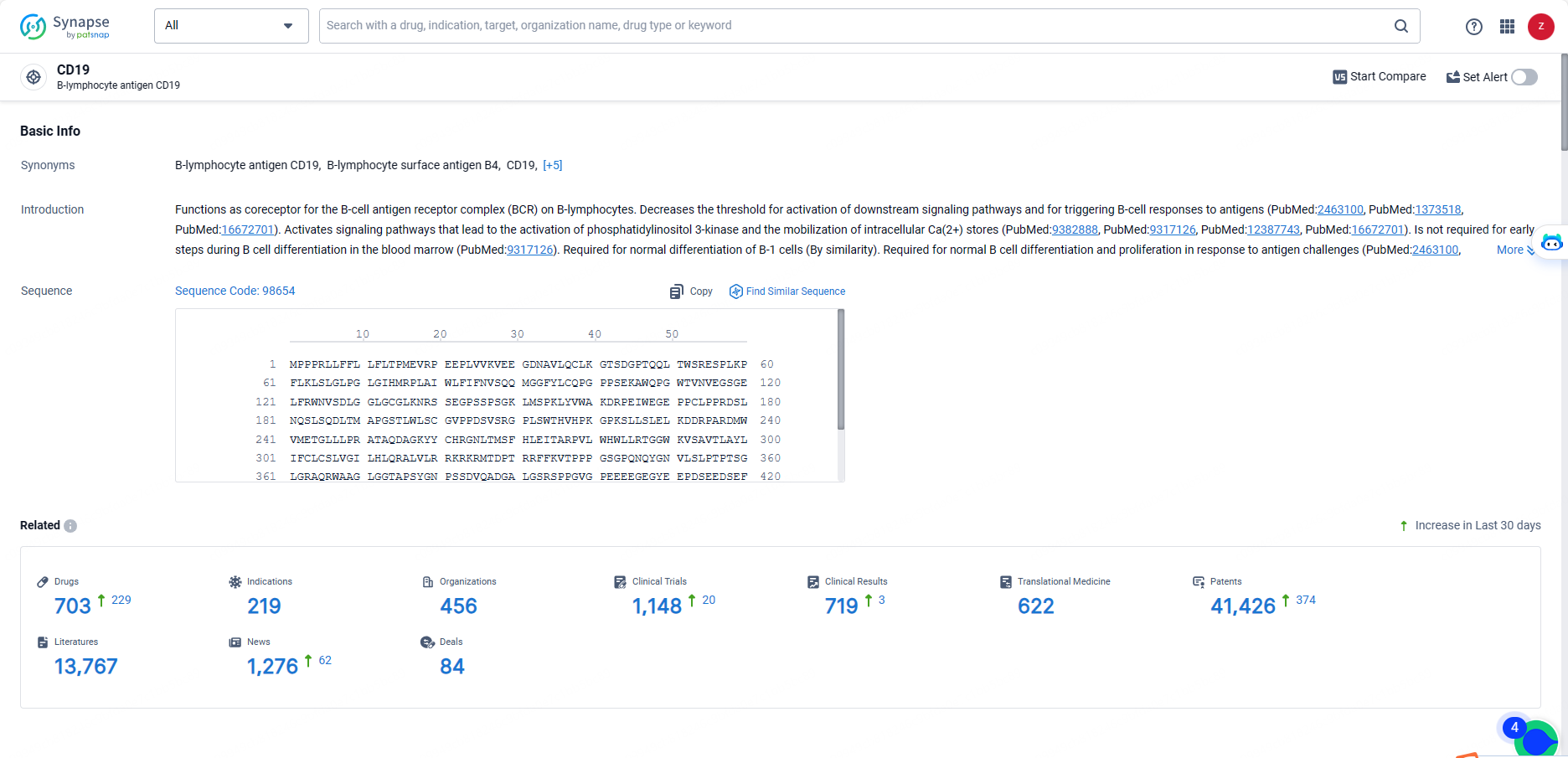
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 20, 2024, there are 703 investigational drugs for the CD19 targets, including 219 indications, 456 R&D institutions involved, with related clinical trials reaching 1148, and as many as 41426 patents.
Lisocabtagene maraleucel is a CAR-T therapy that targets CD19 and is indicated for the treatment of various neoplasms, immune system diseases, hemic and lymphatic diseases, and other related conditions. As a CAR-T therapy, Lisocabtagene maraleucel represents a significant advancement in the treatment of B-cell malignancies. Its approval in Japan marks an important milestone in the global availability of this innovative therapy. The drug's broad range of indications reflects its potential to address unmet medical needs in patients with various types of B-cell lymphomas and leukemias.