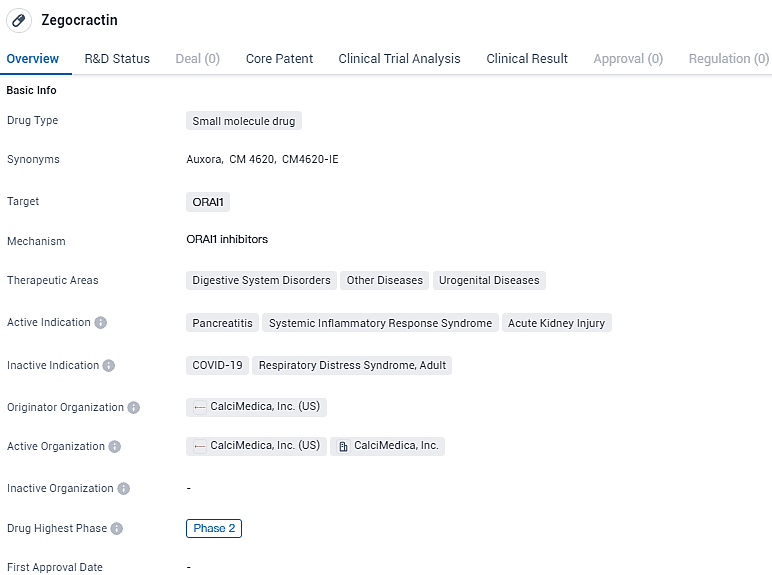
FDA Approves CalciMedica's Phase 2 Trial of Auxora™ for Severe Acute Kidney Injury
CalciMedica, Inc. has disclosed the successful authorization of its Investigational New Drug (IND) application by the FDA. This allows for their principal compound, Auxora™, which acts as a highly effective and discriminatory inhibitor of Orai1-governed CRAC channels, to undergo scrutiny in a Phase 2 clinical study. This examination is targeted at determining the efficacy of the treatment in cases of acute kidney injury compounded by severe acute hypoxemic respiratory failure.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Securing the Investigational New Drug (IND) approval for advancing the Phase 2 research of Auxora in severe Acute Kidney Injury (AKI) represents a pivotal achievement for our team at CalciMedica, as we strive to fulfill the pressing healthcare necessity that these patients encounter," remarked Dr. Rachel Leheny, CEO of the company. "With the implementation of the KOURAGE study, our objective is to evaluate the efficacy of Auxora for individuals with severe AKI and strive to lower the substantial fatality rates linked to this illness."
AKI is differentiated into three stages—1, 2, and 3—based on the severity of renal damage. When Acute Hypoxemic Respiratory Failure (AHRF) is also present, patients with stage 2 and 3 AKI, both considered severe, face a mortality risk of over 50% during hospitalization and within 90 days post-discharge. Individuals who survive severe AKI are at risk of evolving chronic kidney disease, which may necessitate dialysis treatment in the future. In the U.S. alone, there are an estimated 1.1 million individuals enduring stage 2 and 3 AKI, with more than half also dealing with AHRF. No approved treatments for AKI are currently available.
The KOURAGE trial is a controlled clinical study with a design that's randomized, double-blind, and placebo-controlled. It will examine a cohort of 150 patients suffering from stage 2 or 3 AKI with the additional AHRF diagnosis, all of whom are receiving oxygen support ranging from non-invasive mechanical ventilation to high flow nasal delivery and intermittent ventilation when necessary. The stratification of participants will be based on both the specific stage of their AKI and whether they require Intermittent Mandatory Ventilation (IMV). Subjects will either be administered an initial dose of a four-hour Auxora or placebo infusion at 1.25 mL/kg, followed by subsequent doses of Auxora or placebo at 1.0 mL/kg after 24, 48, 72, and 96 hours.
The primary focus of this trial will be patient assessments up to day 30, with the aim to appraise the total duration of survival without the need for ventilators or dialysis. Secondary markers of success will consist of a comprehensive measure that includes overall mortality, the reduction in the estimated rate of glomerular filtration, and the requirement for dialysis during a 90-day span, an assessment referred to as MAKE-90.
"Considering there are currently no pharmaceutical treatments for AKI, our expectation with the KOURAGE study is to demonstrate the potential of Auxora as an innovative treatment path for this urgent healthcare challenge," stated Dr. Sudarshan Hebbar, Chief Medical Officer at CalciMedica.
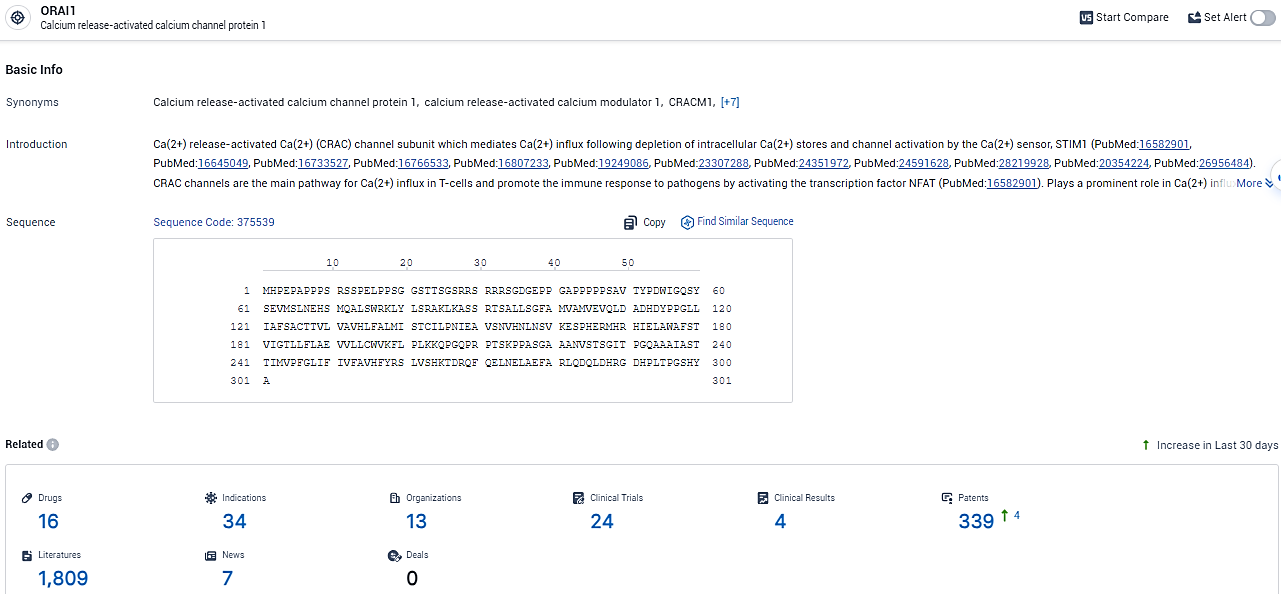
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 20, 2024, there are 16 investigational drugs for the ORAI1 target, including 34 indications, 13 R&D institutions involved, with related clinical trials reaching 24, and as many as 339 patents.
CalciMedica's lead clinical compound, Auxora™, is a potent and selective small molecule inhibitor of Orai1-containing CRAC channels that is being developed for use in patients with acute inflammatory and immunologic illnesses. It is being investigated for its potential use in treating pancreatitis, systemic inflammatory response syndrome, and acute kidney injury. As it is currently in Phase 2 of development, more research is needed to fully understand its effectiveness and safety.