FDA Approves HiFiBiO's IND for BTLA Agonist Antibody HFB200604
HiFiBiO Therapeutics, a biotechnology firm in the clinical stage concentrating on immune modulation, has revealed that the U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for HFB200604. This product, HFB200604, is a leading BTLA agonist monoclonal antibody developed through HiFiBiO's unique Drug Intelligence Science (DIS®) platform. The treatment aims to reinstate immune tolerance in conditions related to inflammation and immunology by activating BTLA signaling in immune cells.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"HFB200604 marks our inaugural autoimmune program entering clinical development, underscoring HiFiBiO’s pioneering strategy in swiftly converting patient insights into effective therapies," stated Liang Schweizer, Ph.D., Founder, Chairperson, and CEO of HiFiBiO Therapeutics. "We are eager to utilize our DIS® platform to tackle the complexities of patient diversity in inflammatory and immunological disorders, aiming for improved response rates."
Francisco Adrian, Ph.D., CSO of HiFiBiO Therapeutics, remarked, "We are excited to introduce our premier BTLA agonist HFB200604 to those affected by inflammatory and immunological conditions. Data from our single-cell immune profiling indicate that BTLA activation may serve as a highly effective method for diminishing immune activation, and we anticipate translating our promising preclinical results into significant clinical advancements."
HiFiBiO intends to initiate a single ascending dose (SAD) Phase 1 clinical trial involving a cohort of healthy volunteers, followed by a multiple ascending dose (MAD) trial in patients diagnosed with specific inflammatory and immunology disorders identified via the HiFiBiO DIS® platform.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
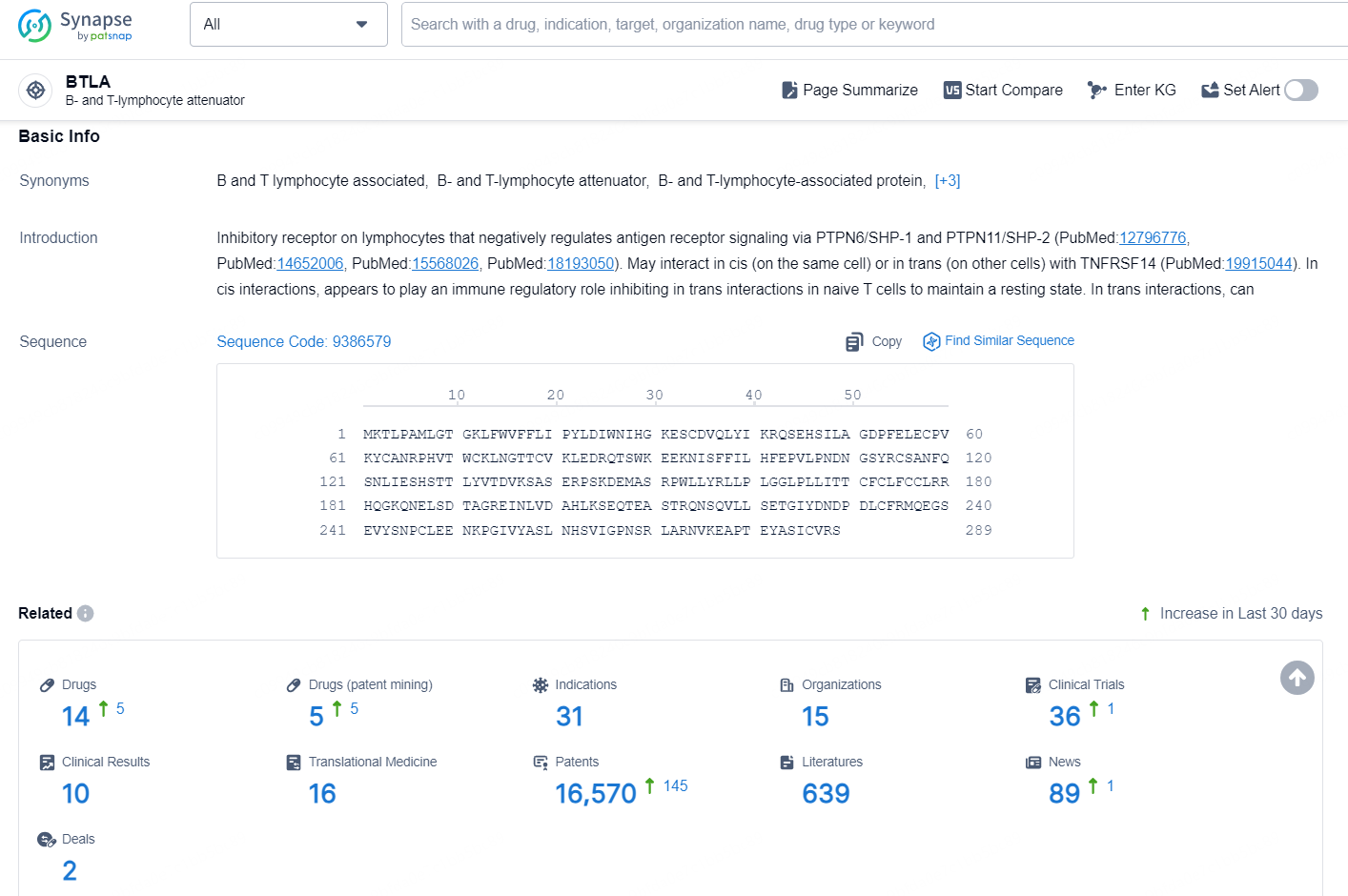
According to the data provided by the Synapse Database, As of November 5, 2024, there are 14 investigational drugs for the BTLA target, including 31 indication, 15 R&D institutions involved, with related clinical trial reaching 36, and as many as 16570 patents.
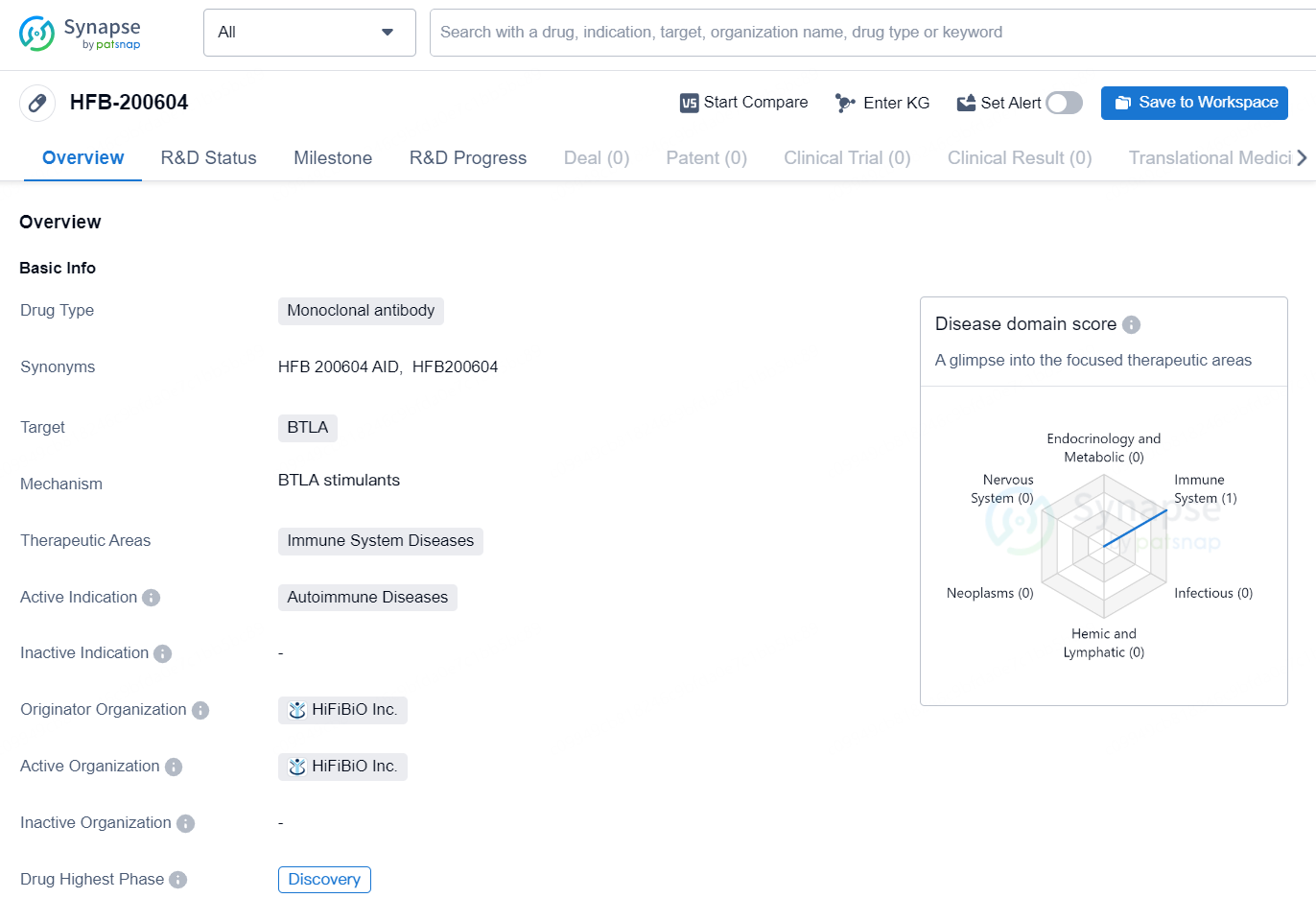
The drug HFB-200604 is a monoclonal antibody that targets BTLA and is intended for the treatment of immune system diseases, with a specific focus on autoimmune diseases. The drug is currently in the Discovery phase and is developed by the organization HiFiBiO Inc. Monoclonal antibodies are a type of biological therapy that can be designed to target specific cells or proteins in the body, in this case, BTLA. BTLA, also known as B and T lymphocyte attenuator, is a protein that plays a role in regulating the immune response. By targeting BTLA, HFB-200604 may have the potential to modulate the immune system and provide therapeutic benefits for individuals with autoimmune diseases.