FDA Approves Jemperli for All Advanced Endometrial Cancer Patients, Marking First Immunotherapy with Proven Survival Benefits
GSK plc revealed that the US Food and Drug Administration granted approval for Jemperli (dostarlimab-gxly) in conjunction with carboplatin and paclitaxel, and subsequently as a monotherapy, for treating adult individuals suffering from primary advanced or recurrent endometrial cancer.
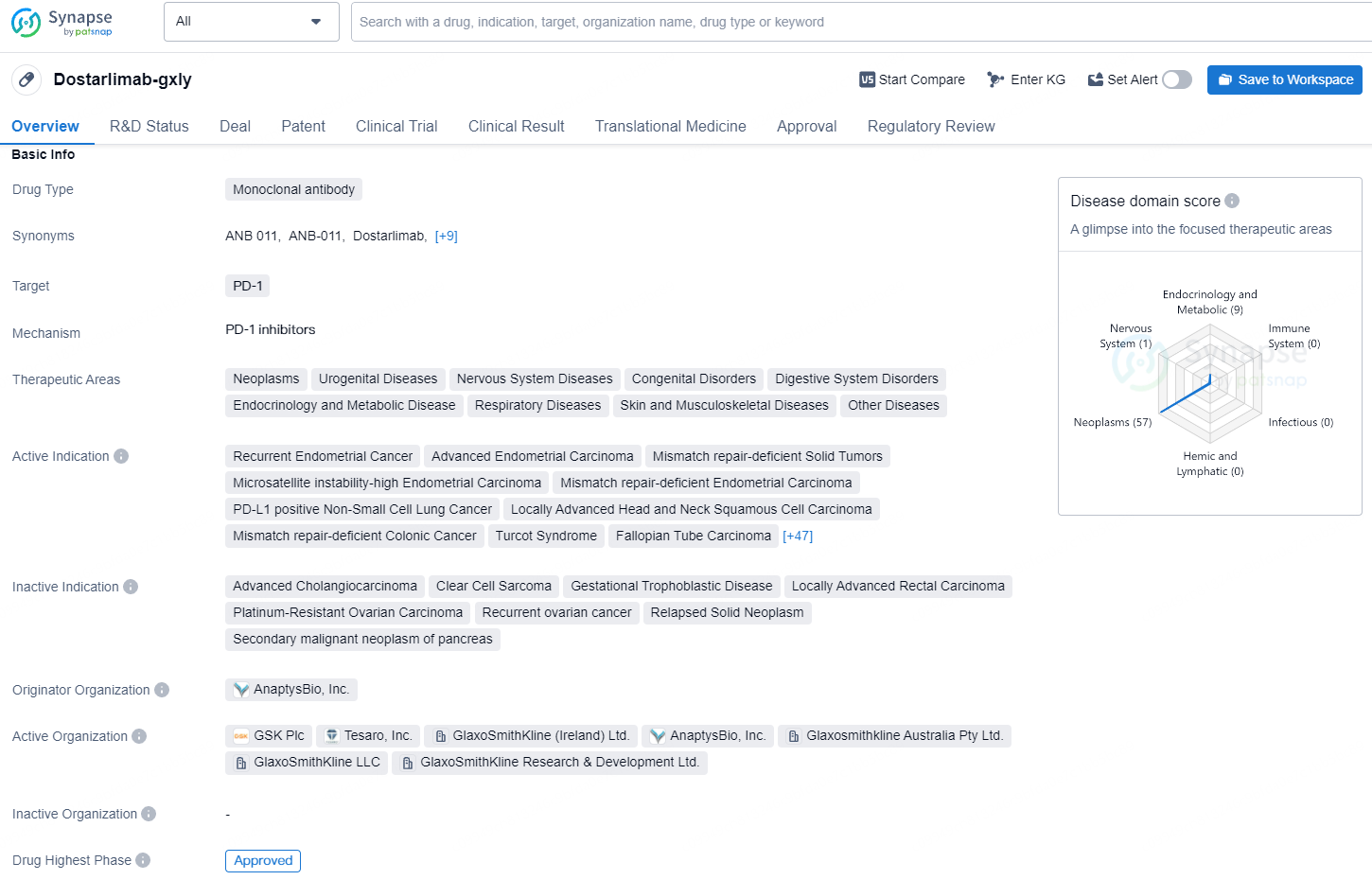
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
This authorization extends the previous use of Jemperli plus chemotherapy to patients with mismatch repair proficient (MMRp) or microsatellite stable tumors, which comprise roughly 70-75% of those diagnosed with endometrial cancer and have few treatment options available. The supplemental Biologics License Application for this expanded use received Priority Review and was granted approval ahead of the Prescription Drug User Fee Act deadline.
Hesham Abdullah, Senior Vice President and Global Head of Oncology R&D at GSK, remarked: "Jemperli plus chemotherapy is the first and sole immuno-oncology regimen to exhibit a notable and meaningful enhancement in overall survival for adult patients with primary advanced or recurrent endometrial cancer, irrespective of biomarker status. We are excited that this treatment is now accessible to more patients in the US, particularly the 70-75% with MMRp/MSS tumors who have had restricted options."
The expanded approval stems from data on the dual primary endpoints of investigator-assessed progression-free survival and overall survival from Part 1 of the RUBY phase III trial. RUBY Part 1 is the only clinical study in this context to demonstrate a statistically significant improvement in overall survival in the comprehensive population of patients with primary advanced or recurrent endometrial cancer, showing a 31% reduction in the risk of death compared to chemotherapy alone.
Matthew Powell, MD, Chief of the Division of Gynecologic Oncology at Washington University School of Medicine and primary investigator of the RUBY trial in the US, commented: "The initial authorization of Jemperli plus chemotherapy revolutionized treatment for patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer. Today’s expanded approval will provide even more patients the opportunity for better outcomes. It is the only immuno-oncology regimen to show a statistically significant overall survival benefit for the entire patient population, which marks a significant advancement in the treatment of this challenging cancer."
Jemperli, an antibody that blocks the programmed death receptor-1 (PD-1), is at the core of GSK’s ongoing immuno-oncology research and development efforts. A comprehensive clinical trial program includes investigations of Jemperli alone and in combination with other therapies in gynecologic, colorectal, and lung cancers, targeting transformative outcomes.
Jemperli was discovered by AnaptysBio, Inc., and licensed to TESARO, Inc., under a collaboration and exclusive licensing agreement signed in March 2014. Under this agreement, GSK oversees the ongoing research, development, commercialization, and manufacturing of Jemperli and cobolimab (GSK4069889), a TIM-3 antagonist.
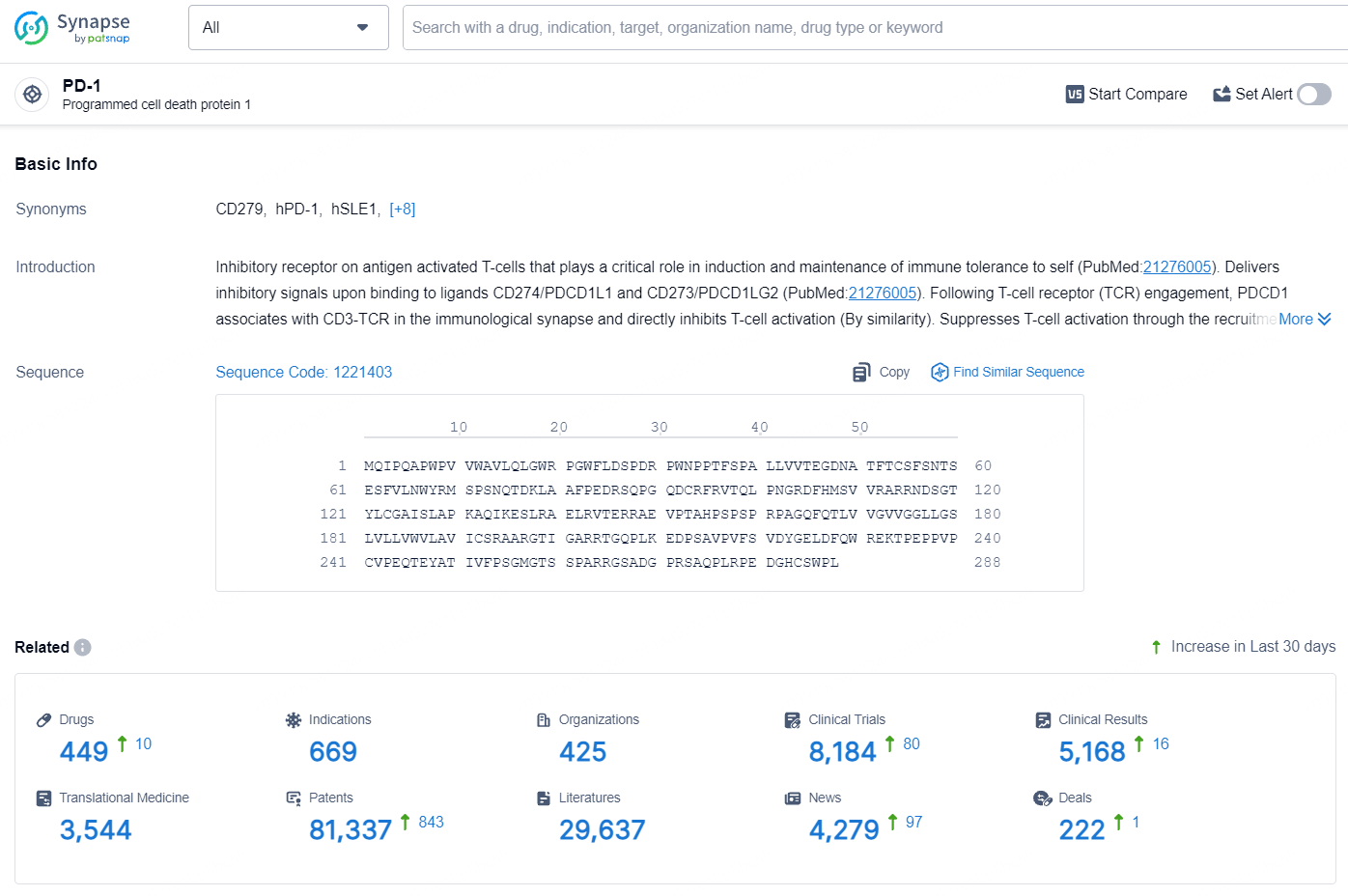
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 7, 2024, there are 449 investigational drugs for the PD-1 target, including 669 indications, 425 R&D institutions involved, with related clinical trials reaching 8184, and as many as 81337 patents.
Dostarlimab-gxly is a monoclonal antibody with a specific target of PD-1, and it has demonstrated efficacy in the treatment of multiple types of cancers. Its approval in multiple countries and its designation for priority review and breakthrough therapy highlight the potential impact of this drug in the pharmaceutical industry and in the treatment of various diseases.