FDA Accelerated Approval for Adaptimmune's TECELRA®, U.S's First Engineered Cell Therapy for Solid Tumors
Adaptimmune Therapeutics plc, a company focusing on cell therapy to revolutionize solid tumor cancer treatment, revealed that the U.S. Food and Drug Administration has granted accelerated approval for TECELRA (afamitresgene autoleucel). This approval is for adults with unresectable or metastatic synovial sarcoma who have previously undergone chemotherapy, are HLA-A*02:01P, -A*02:02P, -A*02:03P, or -A*02:06P positive, and whose tumors express the MAGE-A4 antigen, as confirmed by FDA-approved or cleared companion diagnostic devices.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
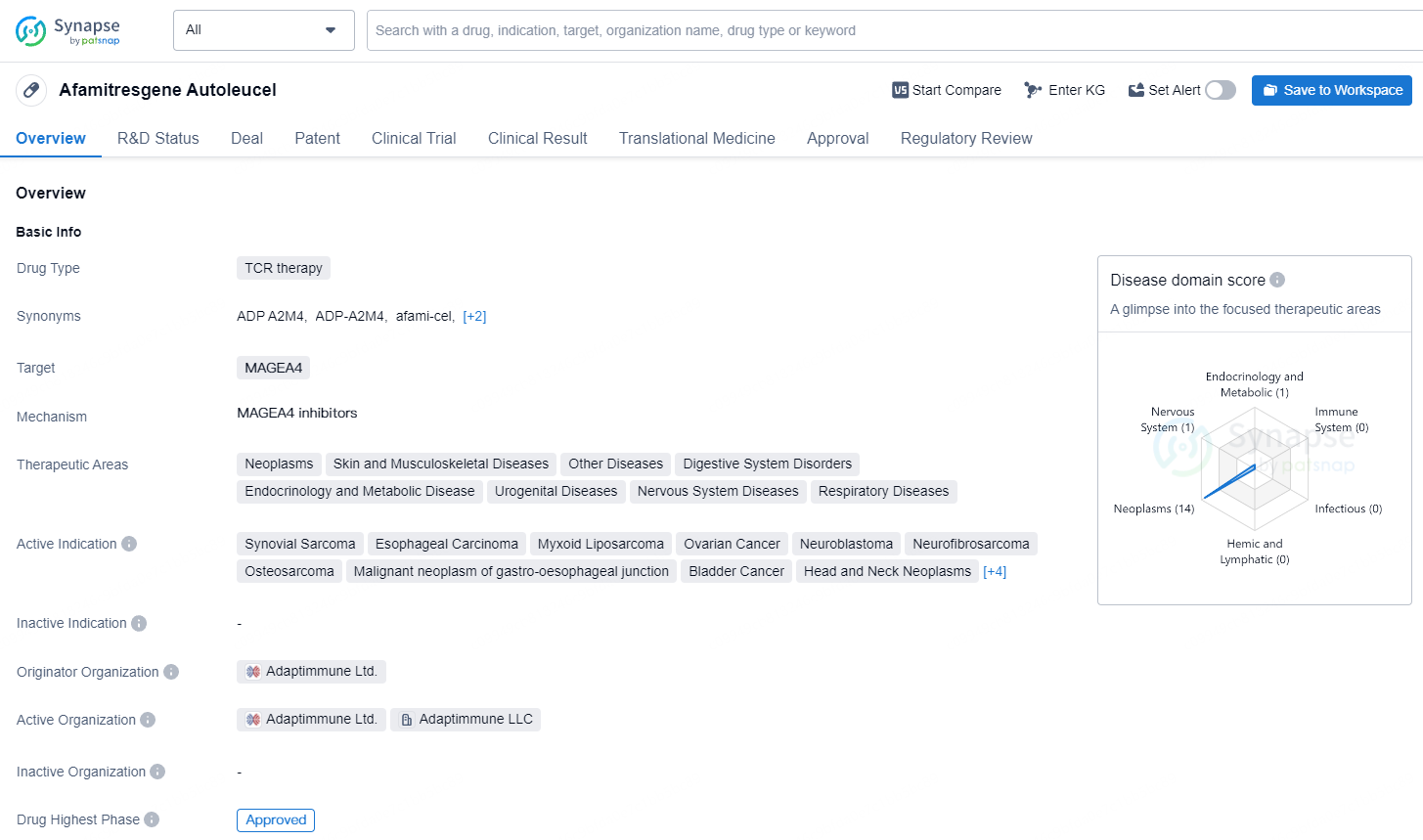 This indication is granted under accelerated approval based on overall response rate and duration of response. Ongoing approval for this indication may be dependent on corroborating and outlining the clinical benefit in a confirmatory study. TECELRA is the inaugural engineered cell therapy approved in the U.S. for a solid tumor cancer and the first new therapeutic option in more than ten years for synovial sarcoma, a rare soft tissue cancer that predominantly affects young adults.
This indication is granted under accelerated approval based on overall response rate and duration of response. Ongoing approval for this indication may be dependent on corroborating and outlining the clinical benefit in a confirmatory study. TECELRA is the inaugural engineered cell therapy approved in the U.S. for a solid tumor cancer and the first new therapeutic option in more than ten years for synovial sarcoma, a rare soft tissue cancer that predominantly affects young adults.
Adrian Rawcliffe, Chief Executive Officer of Adaptimmune: “The approval of TECELRA is a significant milestone in Adaptimmune’s mission to revolutionize cancer treatment and is the result of a decade of innovative R&D. We remain dedicated to advancing our extensive clinical pipeline to assist more patients in need and aim to advance lete-cel, the next late-stage investigational therapy in our sarcoma portfolio, with a rolling BLA submission to the FDA next year.”
The approval of TECELRA was based on data from the SPEARHEAD-1 trial, which included 44 participants. The primary efficacy outcome was the overall response rate assessed by independent review and supported by the duration of response. TECELRA therapy achieved an ORR of 43% with a complete response rate of 4.5%. The median duration of response was 6 months. Among the patients who responded to the treatment, 39% had a response duration of 12 months or longer.
Sandra D’Angelo, MD, Sarcoma Medical Oncologist and Cell Therapist at Memorial Sloan Kettering Cancer Center and Principal Investigator of the SPEARHEAD Trial: “TECELRA (afami-cel), which utilizes each patient's own immune cells to identify and destroy their cancer cells in a one-time infusion treatment, is markedly different from the existing standards of care for advanced synovial sarcoma. This approval provides a critical new option for individuals diagnosed with this sarcoma and marks a significant step forward for the application of cell therapies in solid tumor cancers.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
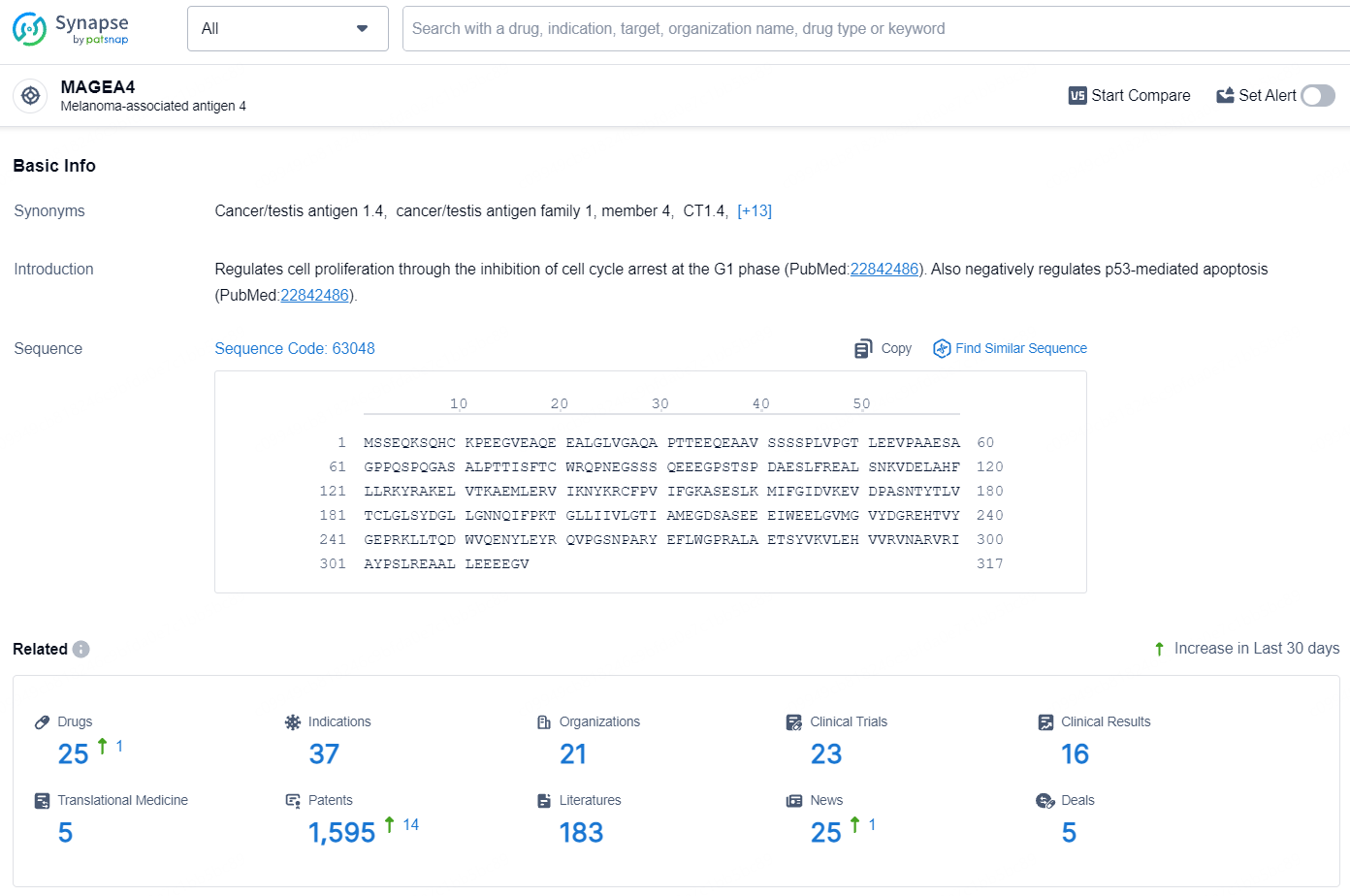 According to the data provided by the Synapse Database, As of August 7, 2024, there are 25 investigational drugs for the MAGEA4 targets, including 37 indications, 21 R&D institutions involved, with related clinical trials reaching 23, and as many as 1595 patents.
According to the data provided by the Synapse Database, As of August 7, 2024, there are 25 investigational drugs for the MAGEA4 targets, including 37 indications, 21 R&D institutions involved, with related clinical trials reaching 23, and as many as 1595 patents.
Afamitresgene autoleucel is a TCR therapy drug developed by Adaptimmune Ltd., targeting MAGEA4 and approved for a wide range of therapeutic areas and active indications. Its first approval was granted in the United States, and it has received several regulatory designations, highlighting its potential to address unmet medical needs in the treatment of various serious or life-threatening conditions.




