Pfizer announces the advancement of the once-daily formulation development for the oral GLP-1 receptor agonist Danuglipron
On July 11th, Pfizer's official website announced that, based on the results of an ongoing pharmacokinetic study (NCT06153758), the company has selected the preferred once-daily extended-release formulation of Danuglipron for further clinical research. Danuglipron is an oral small molecule glucagon-like peptide-1 (GLP-1) receptor agonist. Pfizer plans to conduct dose-optimization studies in the second half of 2024, assessing multiple doses of the preferred extended-release formulation to guide registration-supporting studies. This represents a significant breakthrough for Pfizer in the area of oral small molecule GLP-1R agonists and may also bring new treatment options for patients with obesity.

It is reported that the ongoing open-label, randomized study is evaluating the pharmacokinetics and safety of the extended-release formulation of Danuglipron in healthy adults. So far, the study results have shown a pharmacokinetic profile that supports once-daily dosing, a safety profile consistent with previous Danuglipron studies, including no observed increase in liver enzymes among over 1400 study participants.
Compared to the traditional weekly injection medications Wegovy and Zepbound, the further advancement of Danuglipron's once-daily oral form undoubtedly offers greater convenience for patients.
On December 1st, Pfizer's official website announced the preliminary results of the Phase 2b clinical trial of the oral GLP-1R agonist Danuglipron (PF-06882961) for the treatment of obesity in adults.
This medication is designed to maintain blood sugar levels in a healthy range and works by increasing insulin release and decreasing glucagon release. It also slows down the digestion of food and increases the feeling of fullness after eating.

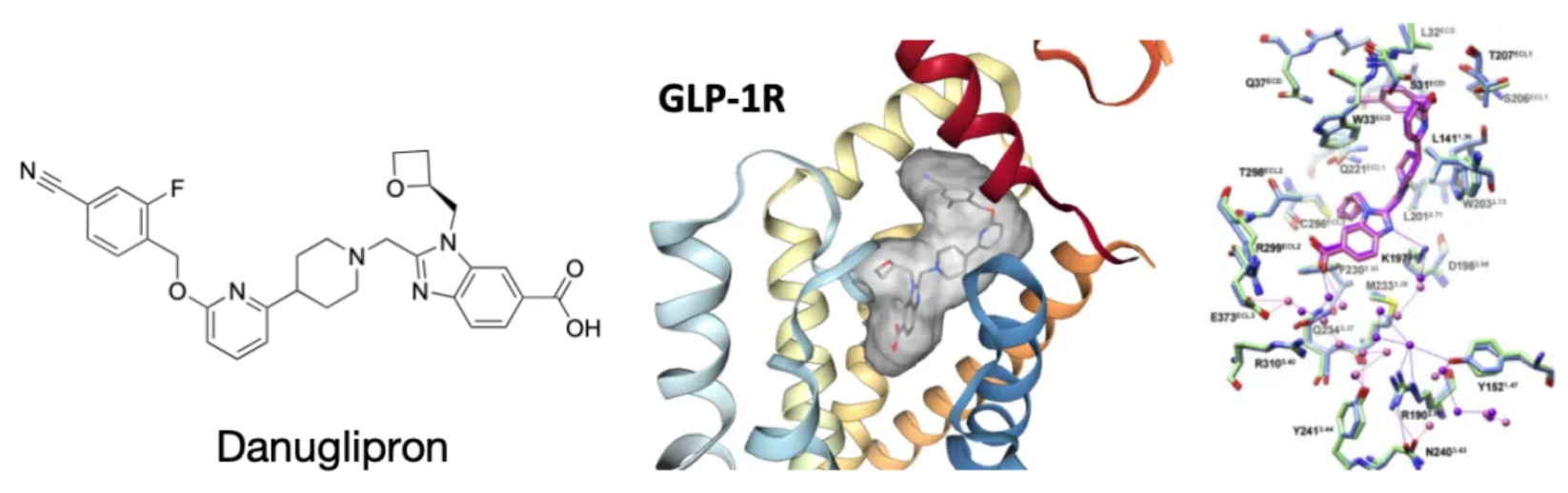
Danuglipron (PF-06882961) is a small molecule agonist targeting the GLP-1R originally developed by Pfizer. The medication is administered orally in tablet form twice daily.
According to Pfizer's latest preliminary results from Phase 2b clinical trials, after adjustment for placebo, the average weight loss rate of Danuglipron at 32 weeks ranged from -8% to -13%, and at 26 weeks it ranged from -5% to -9.5%. In the fiercely competitive GLP-1R agonist field, a weight loss effect of less than 15% to 20% will undoubtedly make the drug's market prospects more uncertain. Pfizer also announced in the first instance that the company will suspend the Phase 3 clinical trial plan for Danuglipron. Instead, it will continue pharmacokinetic studies on the once-daily Danuglipron formulation, the results of which will provide a basis for future clinical development.
Despite previous studies indicating that, compared to the Eli Lilly/Chugai series of small molecule agonists, Danuglipron (PF-06882961) has demonstrated better pharmacological properties that mimic GLP-1 peptides from in vitro activity testing to structural research. Why is it that in clinical studies, Eli Lilly's molecules lead the way, while Pfizer's molecules seem destined for failure?
Following the announcement at the beginning of 2023 that Pfizer would terminate the clinical development of another small molecule agonist, Lotiglipron (PF-07081532), the company's suspension of the Phase 3 clinical trial plan for Danuglipron this time has further exacerbated the situation for the company in the type 2 diabetes and obesity market represented by GLP-1R agonists.
Danuglipron
Danuglipron (PF-06882961) is a small molecule agonist targeting the GLP-1R originally developed by Pfizer. In June 2020, at the American Diabetes Association conference, Pfizer first publicly disclosed clinical Phase I study data on its GLP-1R agonist PF-06882961 for the treatment of diabetes. PF-06882961 has shown significant promise in reducing blood glucose levels and body weight in patients with type 2 diabetes.
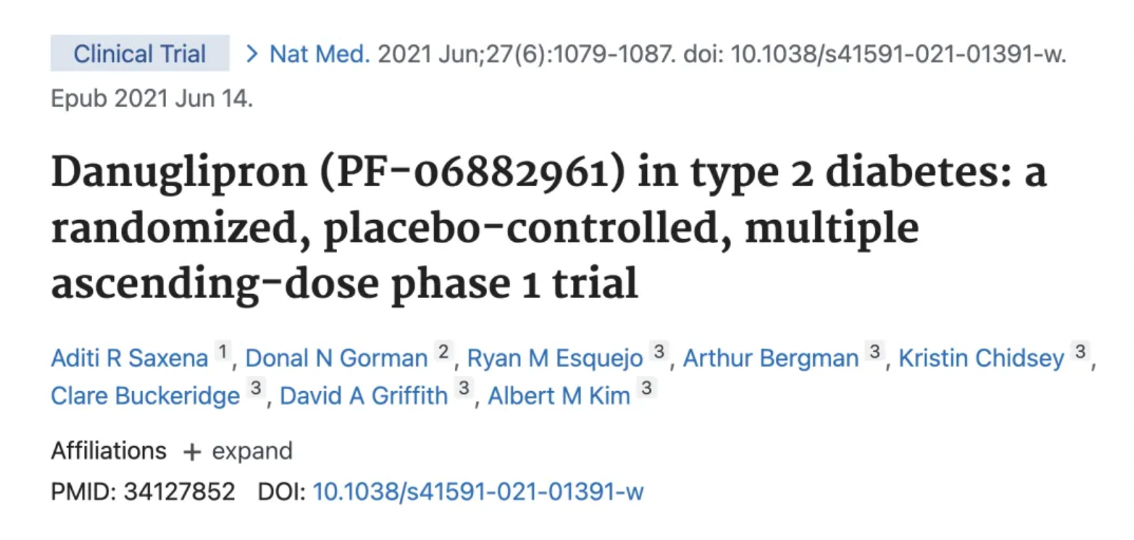
It is reported that in the Phase I study assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics of the drug, 98 patients with type 2 diabetes who had been continuously taking metformin were randomly assigned to receive either a placebo or PF-06882961. Efficacy was improved at all dose levels, but the best results were observed in patients taking the 120 mg dose of the drug. These patients experienced an average weight loss of about 18 pounds, with a reduction in fasting plasma glucose (FPG) of 89.7 mg/dL, and a reduction in the mean daily glucose (MDG) of 105.9 mg/dL. The study was published in the journal Nature Medicine in 2021.
However, prior to Pfizer's announcement of PF-06882961, scientists from Monash University in Australia and researchers from Novo Nordisk took the lead in publishing the high-resolution cryo-EM complex structures of the GLP-1 peptide, PF-06882961, and CHU-128 with the GLP-1R receptor in the journal Molecular Cell. Among them, CHU-128 is a small molecule agonist from a series of compounds patented by Chugai (transferred to Eli Lilly in 2018). The results indicated that, both structurally and in terms of activity data, PF-06882961 is closer to the effects of the GLP-1 peptide than CHU-128.
In 2022, Pfizer officially published a paper in the Journal of Medicinal Chemistry, disclosing the development process of PF-06882961.
Researchers utilized an improved, sensitized cellular activity assay to conduct high-throughput screening of 2.8 million compounds in the presence of the allosteric modulator BETP. Despite the low positive rate of the high-throughput screening (0.013%), a series of less potent hit molecules were identified. Taking compound 2 as an example, researchers optimized it and ultimately obtained the candidate drug PF-06882961, which has good agonistic activity on multiple signaling pathways.
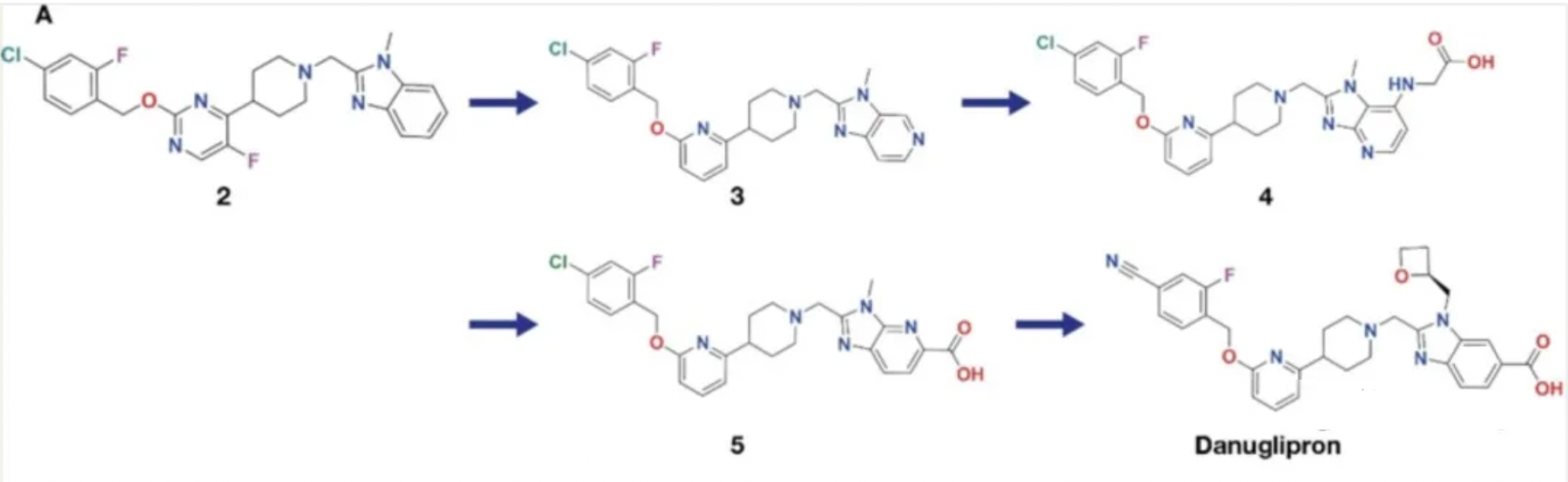
In 2023, Pfizer published research papers successively, reporting the results of randomized clinical trials on the efficacy and safety of PF-06882961 for blood sugar control in patients with type 2 diabetes, the safety, tolerability, pharmacokinetics, and pharmacodynamics of PF-06882961 in Japanese adults with type 2 diabetes from a phase 1 clinical trial, and the results of a 12-week randomized, placebo-controlled, dose-escalation phase 2 trial on the tolerability, safety, and pharmacodynamics of PF-06882961 for the treatment of type 2 diabetes.
Lotiglipron
In June 2023, Pfizer's official website announced that the company would continue to invest resources in the Danuglipron project for subsequent clinical trial research, including the ongoing Phase 2b study. At the same time, the company decided to terminate the clinical development of another small molecule agonist, Lotiglipron.
The decision to terminate the clinical development of Lotiglipron was based on the pharmacokinetic data from the clinical Phase 1 drug-drug interaction studies (NCT05671653 and NCT05788328), as well as the laboratory measurements of elevated transaminases in these Phase 1 studies and the ongoing Phase 2 study (NCT05579977). Although none of these participants reported liver-related symptoms or side effects, there was no evidence of liver function failure, nor was treatment required.

Lotiglipron (PF-07081532) is an oral small molecule GLP-1R agonist co-developed by Sosei Heptares and Pfizer. From the introduction materials of Sosei at the beginning of 2023, it can be seen that Pfizer's PF-07081532, as well as candidate drugs targeting CCR6, MC4R, and CGRP, hold significant positions in Sosei's collaborative pipeline.
This is undoubtedly not good news for Sosei Heptares.
At the end of 2022, Sosei Heptares had announced that its long-term collaboration with Pfizer had advanced the first candidate drug, Lotiglipron (PF-07081532), to Phase 2 clinical trials. The initiation of Phase 2 trials for PF-07081532 would trigger a new milestone payment, from which Sosei Heptares would receive $10 million from Pfizer.
The GLP-1 agonist is the first drug from the collaboration, spanning multiple compounds and therapeutic categories, and was selected for clinical development in 2019. The two companies had referred to PF-07081532 as the next-generation GLP-1 agonist, an oral once-daily treatment to be tested for type 2 diabetes and obesity.
PF-06954522
Following the failure of PF-07081532, on November 6th, Sosei announced that in Pfizer's recently released third-quarter company presentation, Pfizer officially included the PF-06954522 project in the latest Phase 1 clinical study plan. PF-06954522 is being co-developed by Pfizer and Sosei, utilizing Sosei's proprietary technology StaR® during the development process.
In Pfizer's third-quarter company presentation published at the end of October, the author also found the newly listed PF-06954522.
Considering the clinical 1b study data of the small molecule candidate drug GSBR-1290 released by Structure in October, Pfizer's newly launched small molecule project is undoubtedly facing significant external pressure.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!