FDA Approves KEYTRUDA® Combo for Advanced Endometrial Cancer Treatment
Merck, which operates under the name MSD outside the United States and Canada, has revealed that the U.S. Food and Drug Administration has granted approval for KEYTRUDA, the company’s anti-PD-1 therapy, in conjunction with carboplatin and paclitaxel. This regimen is to be followed by KEYTRUDA monotherapy for treating adult patients with advanced or recurrent primary endometrial carcinoma. This latest approval signifies the third indication for endometrial carcinoma and the 40th overall indication for KEYTRUDA in the United States.
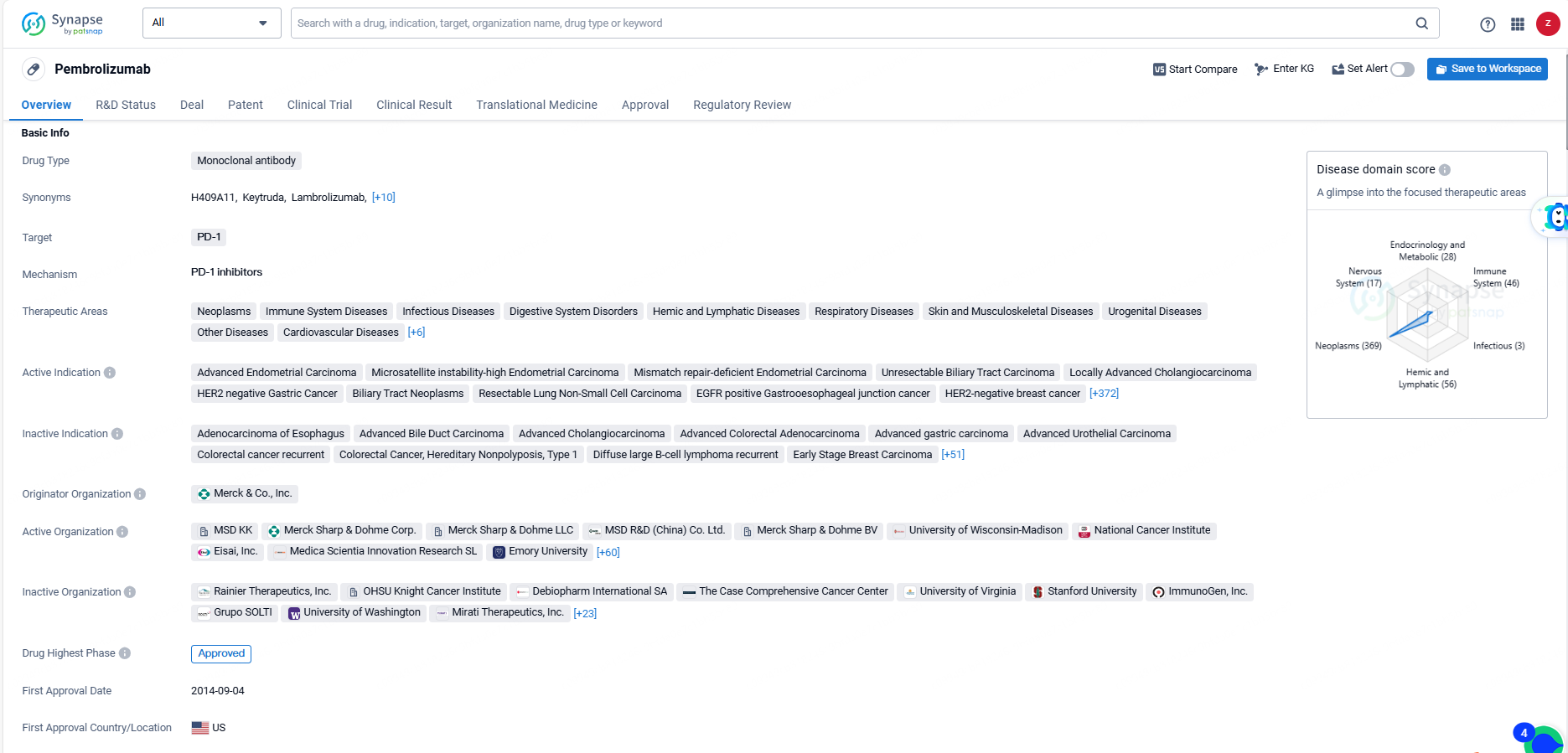
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The green light was granted on the basis of findings from the Phase 3 NRG-GY018 study, also referred to as KEYNOTE-868. Here, the regimen of KEYTRUDA in combination with carboplatin and paclitaxel, followed by KEYTRUDA monotherapy, showed a 40% reduction in the risk of disease progression or death for patients with mismatch repair proficient cancer. For those with mismatch repair deficient cancer, the risk reduction was 70%, when compared to a placebo regime with carboplatin and paclitaxel followed by placebo alone.
"This marks the inaugural Phase 3 trial to statistically assess an anti-PD-1 immunotherapy paired with chemotherapy in both pMMR and dMMR tumor groups as distinct cohorts," stated Dr. Ramez N. Eskander, lead investigator, associate professor in Obstetrics, Gynecology, and Reproductive Services at UCSD School of Medicine, and gynecologic oncologist at Moores Cancer Center, UCSD Health.
Dr. Eskander further commented, "The integration of pembrolizumab with chemotherapy opens a new frontline treatment route for patients with advanced or recurrent endometrial carcinoma, achieving a significantly enhanced and clinically impactful progression-free survival compared to chemotherapy alone, irrespective of mismatch repair status."
The U.S. National Cancer Institute, part of the National Institutes of Health, sponsored this study. NRG Oncology was responsible for designing and leading the trial, backed by funding from NCI along with participation from all National Clinical Trials Network entities. Merck provided financial and logistical support through a Cooperative Research and Development Agreement with NCI.
In the U.S., KEYTRUDA also holds two other approved uses in endometrial carcinoma. The first, based on findings from KEYNOTE-775/Study 309, is in conjunction with LENVIMA (lenvatinib), in partnership with Eisai, for treating adult patients with advanced endometrial carcinoma that is pMMR, as per an FDA-approved test, or not microsatellite instability-high, who have seen disease progression after previous systemic therapy in any context and are not eligible for curative surgery or radiation.
The second approved use, based on KEYNOTE-158, involves KEYTRUDA as a standalone treatment for adult patients with advanced endometrial carcinoma that is MSI-H or dMMR, determined through an FDA-approved test, who have experienced disease progression after previous systemic therapy in any context and are not candidates for curative surgery or radiation.
This approval underwent review under Project Orbis, an initiative by the FDA Oncology Center of Excellence that streamlines simultaneous review of oncology drugs with international collaborators. As part of this initiative, the NRG-GY018/KEYNOTE-868 application is under consideration by regulatory bodies in Israel, Canada, Australia, Singapore, Brazil, and the United Kingdom.
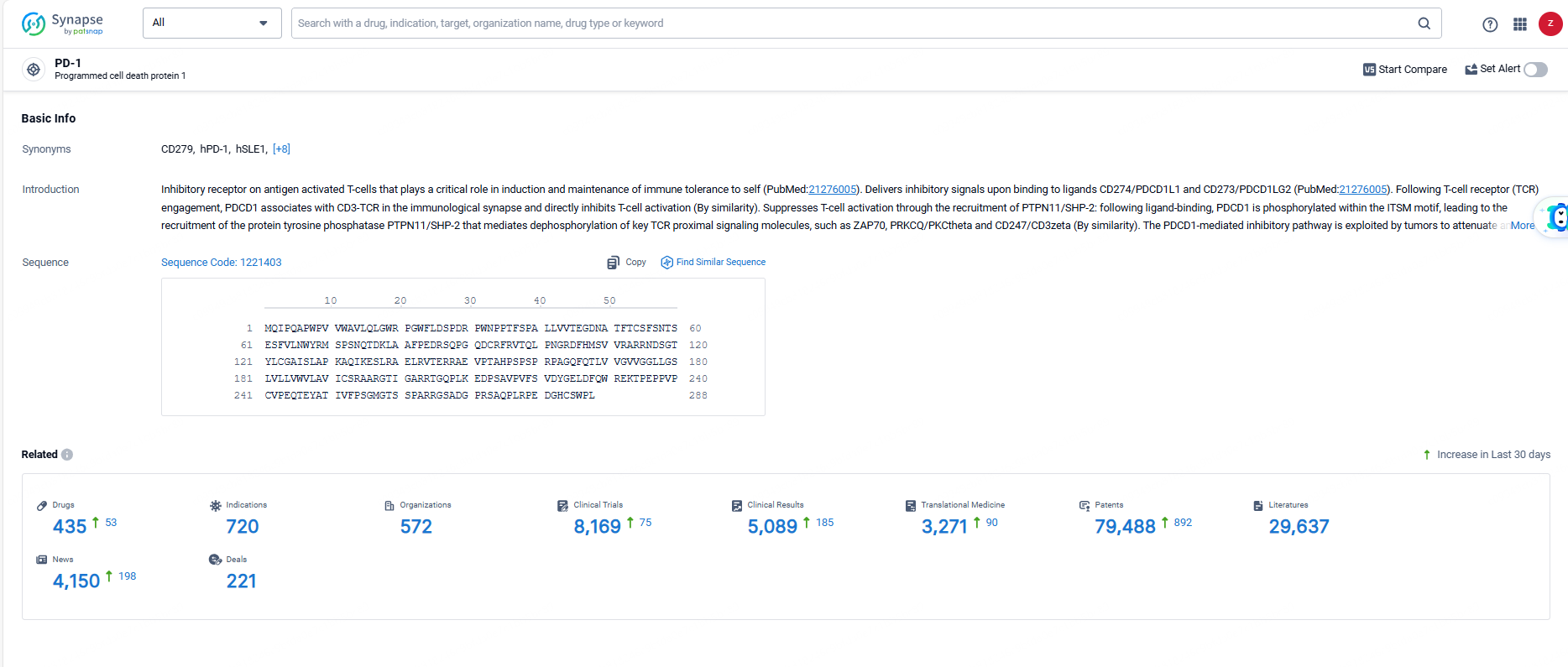
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 23, 2024, there are 435 investigational drugs for the PD-1 target, including 720 indications, 572 R&D institutions involved, with related clinical trials reaching 8169, and as many as 79488 patents.
Pembrolizumab is a monoclonal antibody drug that targets PD-1 and is used in the treatment of a wide range of therapeutic areas including neoplasms, immune system diseases, infectious diseases, digestive system disorders, and various others. The drug's approval in both the global and Chinese markets signifies its potential to benefit a wide range of patient populations worldwide.