FDA Approves Oricell's IND Application for Innovative CAR-T Therapy, OriCAR-017
Oricell Therapeutics, a firm at the clinical development phase in the biotech industry, declared that its application for the exploration of a new pharmaceutical, OriCAR-017, has received approval from the FDA in the United States for usage in individuals suffering from multiple myeloma that is recurrent or resistant to treatment.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.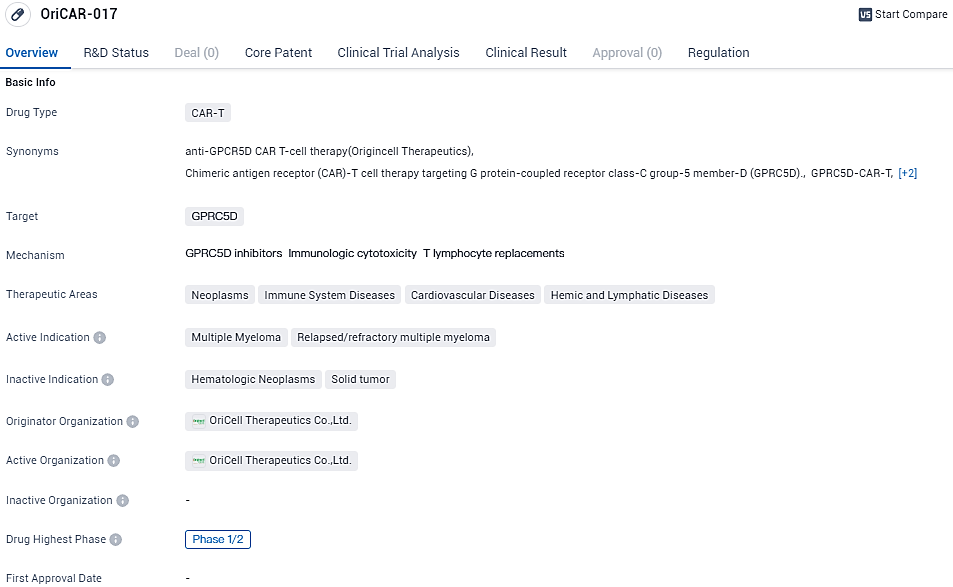
OriCAR-017 stands as a novel therapeutic approach, framed as a chimeric antigen receptor T cell treatment, and is specifically devised to address GPRC5D. Utilizing Oricell's cutting-edge technologies, which include the Ori®Ab antibodies, the Ori®CAR designs, and a distinctive competence in CMC processes, this therapy ensures an enhanced attachment and elevated effectiveness in both persistence and combating tumor cells through revitalized CAR-T cells. Through the issuance of the IND, Oricell now possesses the authorization to commence the introductory phases of clinical trials for OriCAR-017 within the US territory without delay.
The United States Food and Drug Administration (FDA) bestowed an IND clearance for OriCAR-017, a milestone following its previous sanction by the NMPA in 2023. Compelling clinical findings, stemming from an Investigator-Initiated Trial, were disseminated at the 2022 ASCO, the 2022 EHA, and within The Lancet Haematology. This evidence revealed compelling responses amongst the cohort of ten patients dealing with R/R MM, as adjudicated by the IMWG norms. The therapy yielded an unparalleled 100% rate of overall response, with a significant 80% achieving a stringent complete response. Impressively, a 100% negative rate for minimal residual disease was recorded on day 28, with successive validation at the 3-month milestone.
Remarkably, the treatment regimen manifested a high safety margin, evidenced by the absence of Immune effector cell-associated neurotoxicity syndrome, cerebellar complications, or procrastinated infections. Moreover, incidents of CRS were confined to Grade 1 and Grade 2 classifications and demonstrated swift resolution. Within the group of ten patients afflicted with R/R MM, a varied clinical backdrop was noted: 40% presented with extramedullary disease; half underwent previous treatments involving BCMA CAR-T; 70% were identified with high-risk cytogenetics; and a similar proportion presented with an ECOG performance status of 2, with 80% classified within ISS stages II and III.
Peter He, co-creator and the principal scientific leader at Oricell, expressed profound optimism towards the impactful safety, effectiveness, and endurance profiles of OriCAR-017, heralding its potential for global patient benefit within the multiple myeloma landscape. He also underscored the culmination of a decade's research and development efforts that have not only materialized OriCAR-017 but also fortified a robust, integrated platform. This platform is integral to the creation of uniquely innovative CAR-T cell products targeting both hematologic and solid malignancies.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!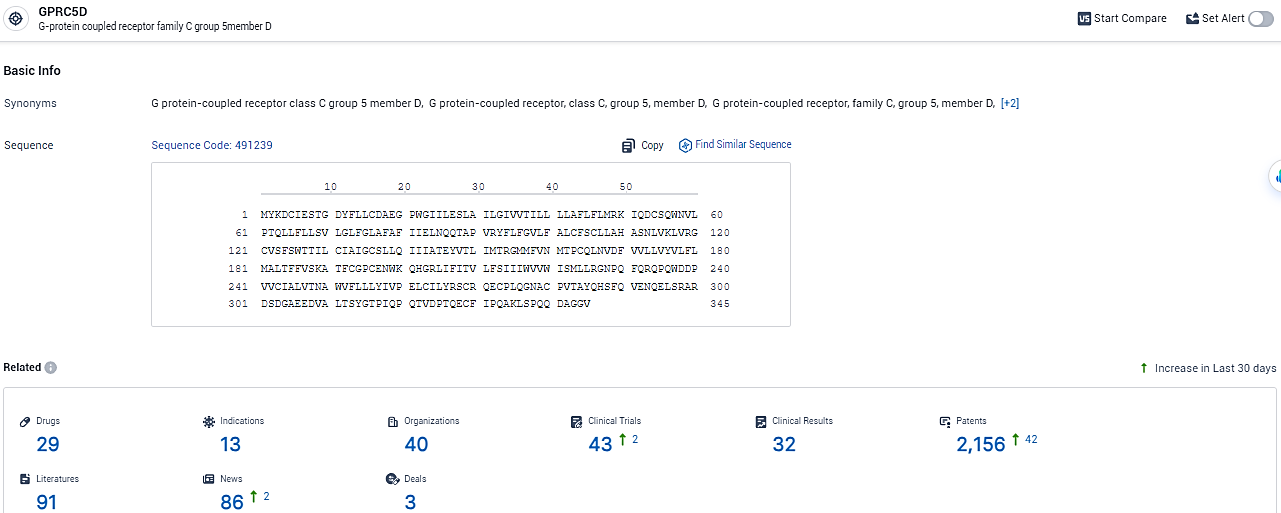
According to the data provided by the Synapse Database, As of February 5, 2024, there are 29 investigational drugs for the GPRC5D target, including 13 indications, 40 R&D institutions involved, with related clinical trials reaching 43, and as many as 2156 patents.
OriCAR-017 targets GPRC5D and aims to treat multiple myeloma and relapsed/refractory multiple myeloma. The drug is currently in Phase 1/2 of clinical development globally and in China. It has been designated as an orphan drug, indicating its potential to address unmet medical needs in rare diseases.