Sarepta Therapeutics' SRP-5051 Shows Promise in MOMENTUM Phase 2 Trial for Duchenne Muscular Dystrophy
Sarepta Therapeutics, Inc., a frontrunner in the field of precision genetic therapy targeting uncommon illnesses, has disclosed encouraging results from the second segment of its international Phase 2 MOMENTUM trial. This significant multi-ascending dose study is evaluating the efficacy of SRP-5051 (vesleteplirsen) in participants aged between 8 and 21 years. Designed as a cutting-edge peptide phosphorodiamidate morpholino oligomer (PPMO), SRP-5051 is being developed specifically for individuals with Duchenne muscular dystrophy (DMD) who have the potential to benefit from exon 51 skipping therapy.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.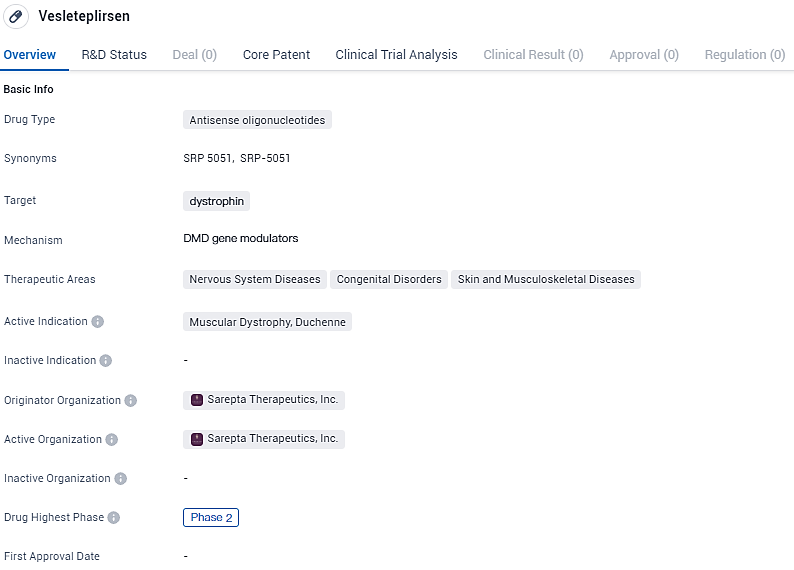
Research from the latter phase of the MOMENTUM trial indicated that the higher intended dosage of SRP-5051, administered at roughly 30 mg/kg every month, led to an average increase in dystrophin levels by 5.17% and facilitated exon skipping by an average of 11.11% at the 28-week marker. During the trials, instances of low magnesium levels were observed in participants receiving SRP-5051; however, this was counteracted and consistently overseen with the help of preventive magnesium intake as delineated by the research guidelines.
Louise Rodino-Klapac, Ph.D., who is the EVP, CSO, and spearhead of R&D at Sarepta Therapeutics, noted, “Administering SRP-5051 on a monthly basis has demonstrated a significant elevation in both dystrophin production and exon skipping when contrasted with the effects of weekly administrations of eteplirsen.” She added, “With our leadership position in Duchenne research, we at Sarepta are highly focused on developing impactful therapies for those affected by Duchenne and various other rare disorders that currently lack effective treatments.”
“The increase in dystrophin production we've seen with SRP-5051 in the MOMENTUM research holds promise,” mentioned Eugenio Mercuri, M.D., Ph.D., leader of the Neuromuscular Division at the Catholic University in Rome and a collaborator in the investigation. “Moreover, with rigorous supplementation and oversight, we've observed no further complications related to the hypomagnesemia condition.” He observed, “Based on our findings, SRP-5051 shows potential for significant impact as a treatment strategy for Duchenne patients who have a specific mutation that makes them suitable for exon 51 skipping.”
In the MOMENTUM Part B study, participants reported seven serious adverse events linked to the treatment. There were four instances of serious hypomagnesemia and three of serious hypokalemia. The occurrence of hypomagnesemia had been documented in prior clinical evaluations of SRP-5051 and, in this phase of MOMENTUM, subjects received magnesium as a preventative measure. Notably, there were no discontinuations of treatment owed to related adverse effects throughout the study.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!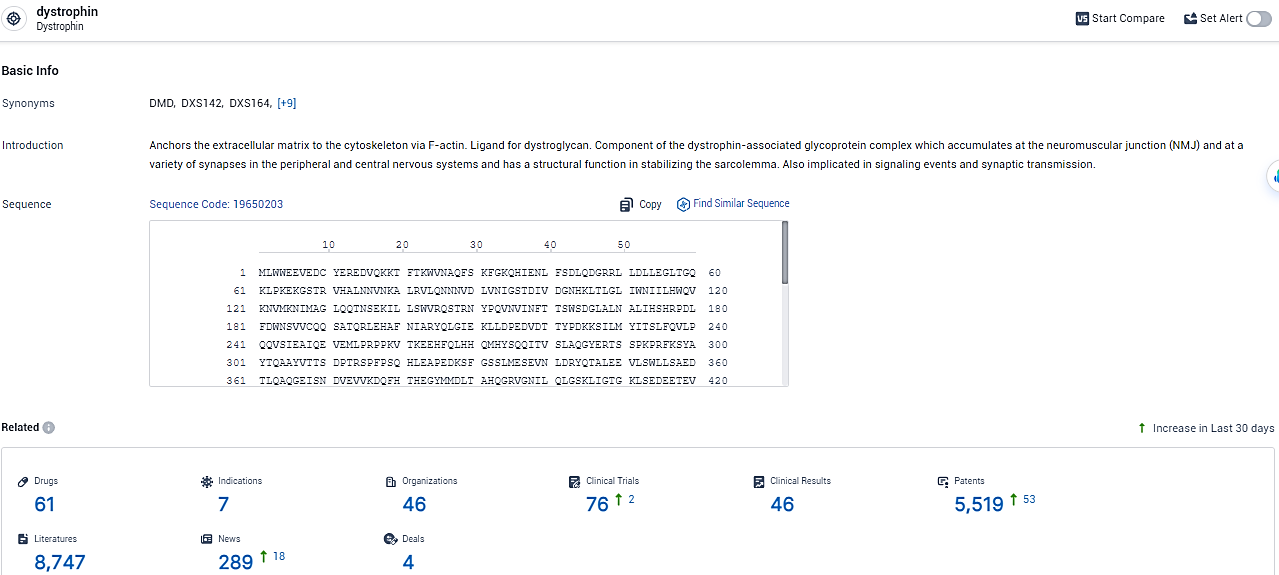
According to the data provided by the Synapse Database, As of February 5, 2024, there are 61 investigational drugs for the dystrophin target, including 7 indications, 46 R&D institutions involved, with related clinical trials reaching 76, and as many as 5519 patents.
SRP-5051 is an investigational agent using Sarepta’s PPMO chemistry and exon-skipping technology to skip exon 51 of the dystrophin gene. SRP-5051 is being developed for the treatment of Muscular Dystrophy, specifically Duchenne. The drug is currently in Phase 2 of clinical development, indicating that it has shown promise in early testing. The development of Vesleteplirsen represents a potential advancement in the treatment of Duchenne Muscular Dystrophy, a debilitating genetic disorder.




