U.S. FDA Clears Takeda's GAMMAGARD LIQUID® for Chronic Inflammatory Nerve Disorder Treatment in Adults
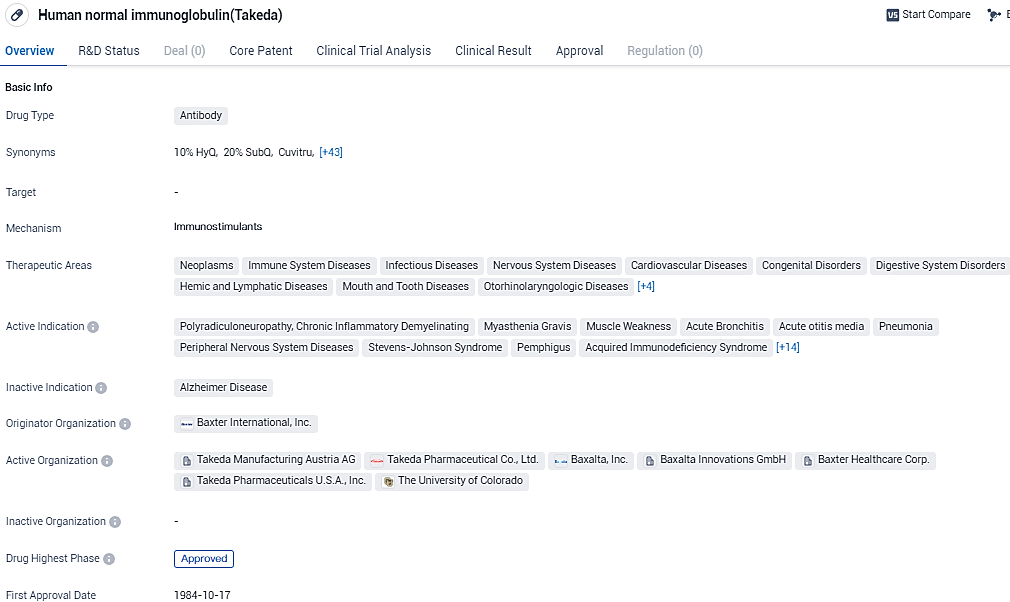
Takeda has disclosed that GAMMAGARD LIQUID® [Immune Globulin Infusion (Human) 10% solution], their intravenous immunoglobulin treatment, has received authorization from the U.S. Food and Drug Administration. This medication is specifically designed to ameliorate neuromuscular dysfunction and mitigate deficits in adults suffering from chronic inflammatory demyelinating polyneuropathy.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
GAMMAGARD LIQUID®, a therapeutic plasma product, may be prescribed as a starting treatment involving an initial dose coupled with ongoing maintenance doses. In the context of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), research on GAMMAGARD LIQUID has neither covered patients who have never received immunoglobulin nor extended maintenance treatment beyond a half-year duration.
This advancement coincides with the recent U.S. FDA endorsement of HYQVIA® [Immune Globulin Infusion 10% with Recombinant Human Hyaluronidase] for sustained treatment to forestall recurrence of neuromuscular deterioration and functional deficits in adult CIDP sufferers. Unique in its combination, HYQVIA merges immunoglobulin with hyaluronidase to provide a supportive subcutaneous IG infusion.
Takeda's high-ranking official, Richard Ascroft, who oversees the U.S. Plasma-Derived Therapies sector, expressed, “The sanctioning of GAMMAGARD LIQUID for CIDP treatment reinforces our longstanding dedication to enhance plasma-based treatments for individuals with rare neuromuscular conditions, as well as to deliver our innovative IG treatments to this patient group. With the concurrent authorization of HYQVIA in the States, we’re in a position to present both induction and continuous treatment alternatives that can suit the personal health regimens of adults with CIDP.”
“IG therapy, considered the benchmark treatment for CIDP, is believed to restore abnormal immune function via immunomodulation,” declared Dr. Mamatha Pasnoor, a neuroscience faculty member at the University of Kansas Medical Center. “Given the progressive and intricate nature of CIDP, a spectrum of treatment modalities is essential. Healthcare providers now have access to an extra treatment avenue aimed at aiding adult CIDP patients in controlling their condition.”
In the United States, GAMMAGARD LIQUID stands as the sole Intravenous Immunoglobulin (IVIG) with approval for multiple neuromuscular conditions, including CIDP and as the exclusive FDA-sanctioned IVIG for treating multifocal motor neuropathy by sustaining muscle force and reducing impairment in adult patients. Additionally, it is approved as a substitution treatment for individuals aged two and above who are diagnosed with primary immunodeficiency.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this indications by clicking the image link below. Dive in to gain deeper insights!
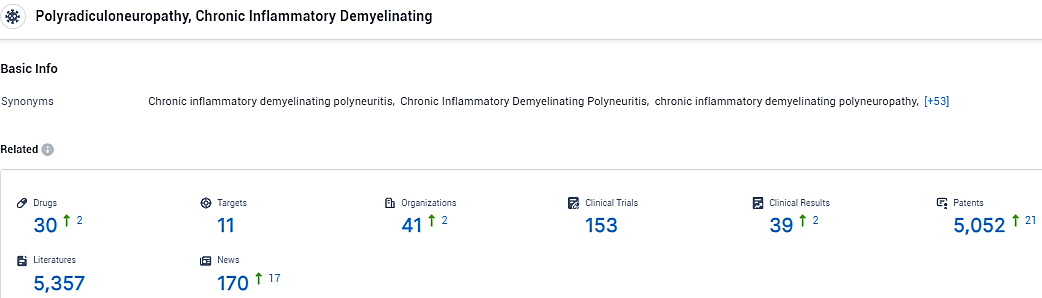
According to the data provided by the Synapse Database, As of February 5, 2024, there are 30 investigational drugs for the Chronic inflammatory demyelinating polyneuritis, including 11 targets, 41 R&D institutions involved, with related clinical trials reaching 153, and as many as 5052 patents.
GAMMAGARD LIQUID[Immune Globulin Infusion (Human) 10% solution] is an intravenous immunoglobulin that is infused into the veins. GAMMAGARD LIQUID is approved in the U.S. as an IG therapy to improve neuromuscular disability and impairment in adult patients with CIDP, as a replacement therapy for primary immunodeficiency in adult and pediatric patients two years of age and older, and as a maintenance therapy to improve muscle strength and disability in adult patients with multifocal motor neuropathy. Also known as KIOVIG outside the U.S. and Canada, it is approved in 66 countries worldwide.