FDA Approves Rgenta Therapeutics' IND for RGT-61159, an Oral RNA Modulator Targeting ACC and CRC
Rgenta Therapeutics, a biotech company at the forefront of creating innovative oral small molecule therapies that target RNA and RNA regulation, has received clearance from the U.S. Food and Drug Administration for its Investigational New Drug application for RGT-61159. This new treatment is being developed for possible use against adenoid cystic carcinoma, colorectal cancer, and other solid tumors, as well as acute myeloid leukemia.
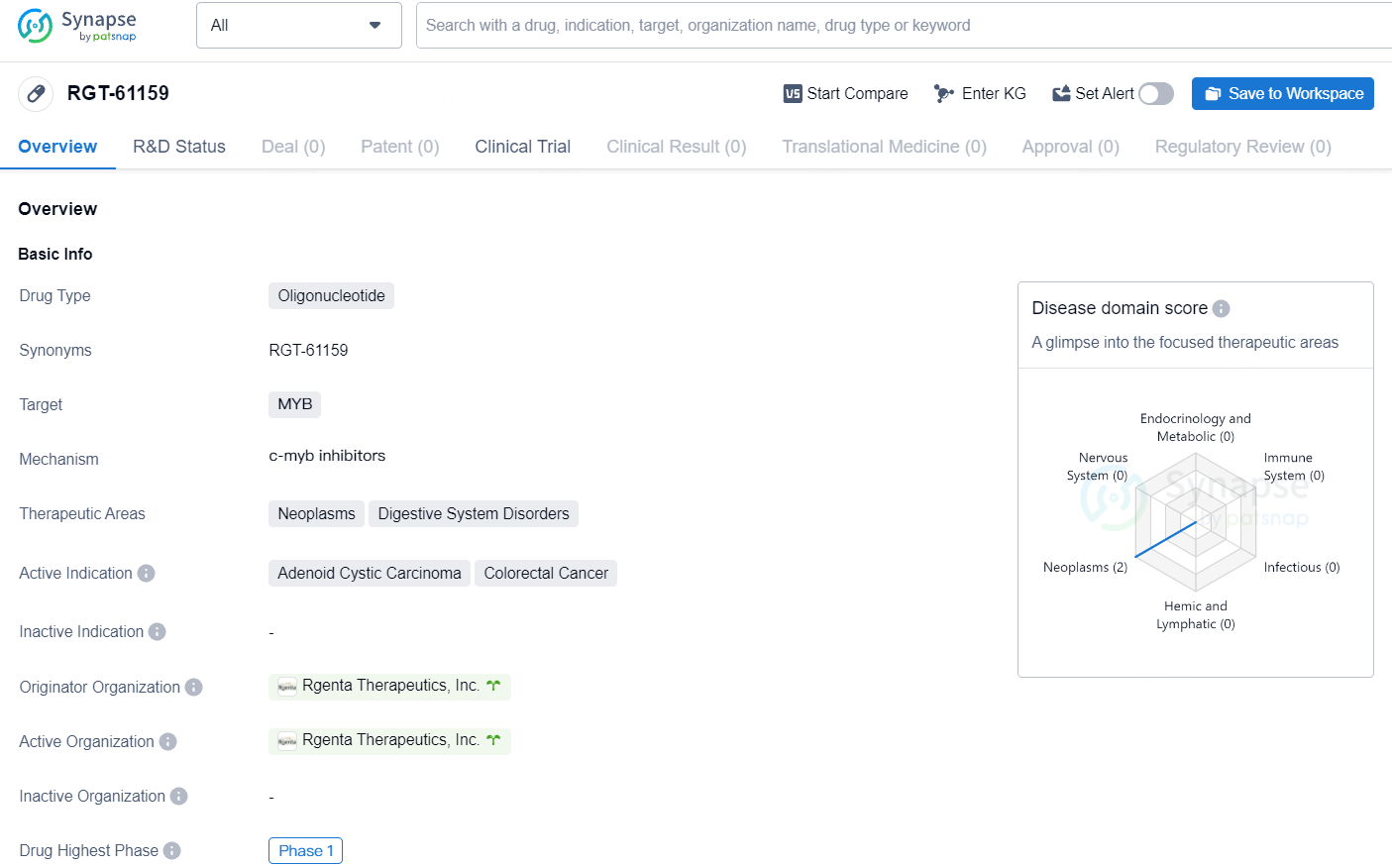
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The approval of our initial IND application marks a pivotal step in Rgenta’s pursuit of developing oral, small molecule RNA-targeting therapies for diseases previously deemed untreatable," stated Simon Xi, Ph.D., co-founder and CEO of Rgenta. "We are eager to commence clinical trials of RGT-61159, beginning with a Phase 1a/1b study in adult patients suffering from ACC and CRC, with the aim to offer a novel treatment option for these challenging cancers."
"Developing small molecule drugs that target oncogenic drivers, such as MYB, has been historically difficult," noted Travis Wager, Ph.D., co-founder, president, and chief scientific officer. "RGT-61159 has shown strong inhibition of oncogenic MYB protein production and significant tumor growth reduction at tolerated doses in preclinical ACC and other cancer models. We are thrilled to advance this innovative therapy into clinical trials."
RGT-61159 is an orally administered small molecule specifically designed to modulate the splicing of the MYB transcription factor, leading to decreased production of the oncogenic MYB protein. This reduction has the potential to inhibit the proliferation or induce apoptosis of cancer cells overexpressing MYB protein.
MYB functions as a key regulator of cell proliferation and differentiation processes, with aberrant expression observed in various human cancers including adenoid cystic carcinoma, acute myeloid leukemias, T-cell acute lymphoblastic leukemias, colorectal cancer, small cell lung cancer, and breast cancer.
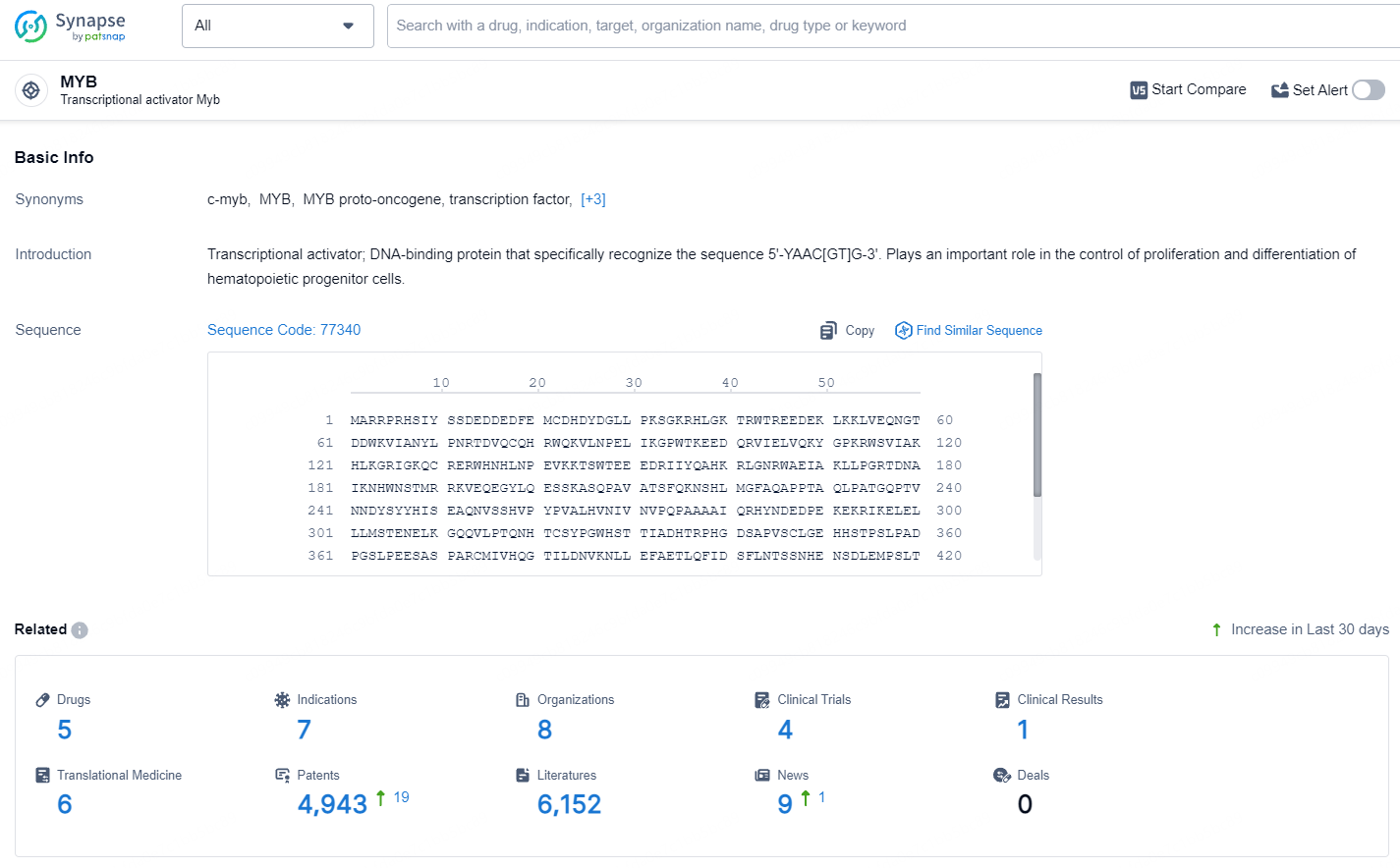
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 15, 2024, there are 5 investigational drugs for the MYB targets, including 7 indications, 8 R&D institutions involved, with related clinical trials reaching 4, and as many as 4943 patents.
RGT-61159 is an oral small molecule RNA modulator that selectively targets MYB RNA and inhibits the production of oncogenic MYB protein, developed by Rgenta Therapeutics. In preclinical models, RGT-61159 exhibits effective inhibition of oncogenic MYB protein production and significantly inhibits tumor growth at tolerated doses. With the advancement of clinical trials, the efficacy and safety of RGT-61159 will be further validated. If clinical trials are successful, RGT-61159 is expected to become a new option for treating various cancers.