FDA Approves SystImmune's IND Application for BL-M11D1 in Relapsed Acute Myeloid Leukemia
SystImmune, Inc. (SystImmune), a biotechnology firm in the clinical stage, has reported that the U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for BL-M11D1, an antibody-drug conjugate (ADC) that targets CD33, a protein found on myeloid cells. This IND approval facilitates the commencement of a Phase 1 clinical trial, designated BLM11D1-HM-101, aimed at assessing the safety, tolerability, pharmacokinetics, and initial efficacy of BL-M11D1 for treating patients with relapsed or refractory Acute Myeloid Leukemia (AML) in the U.S.
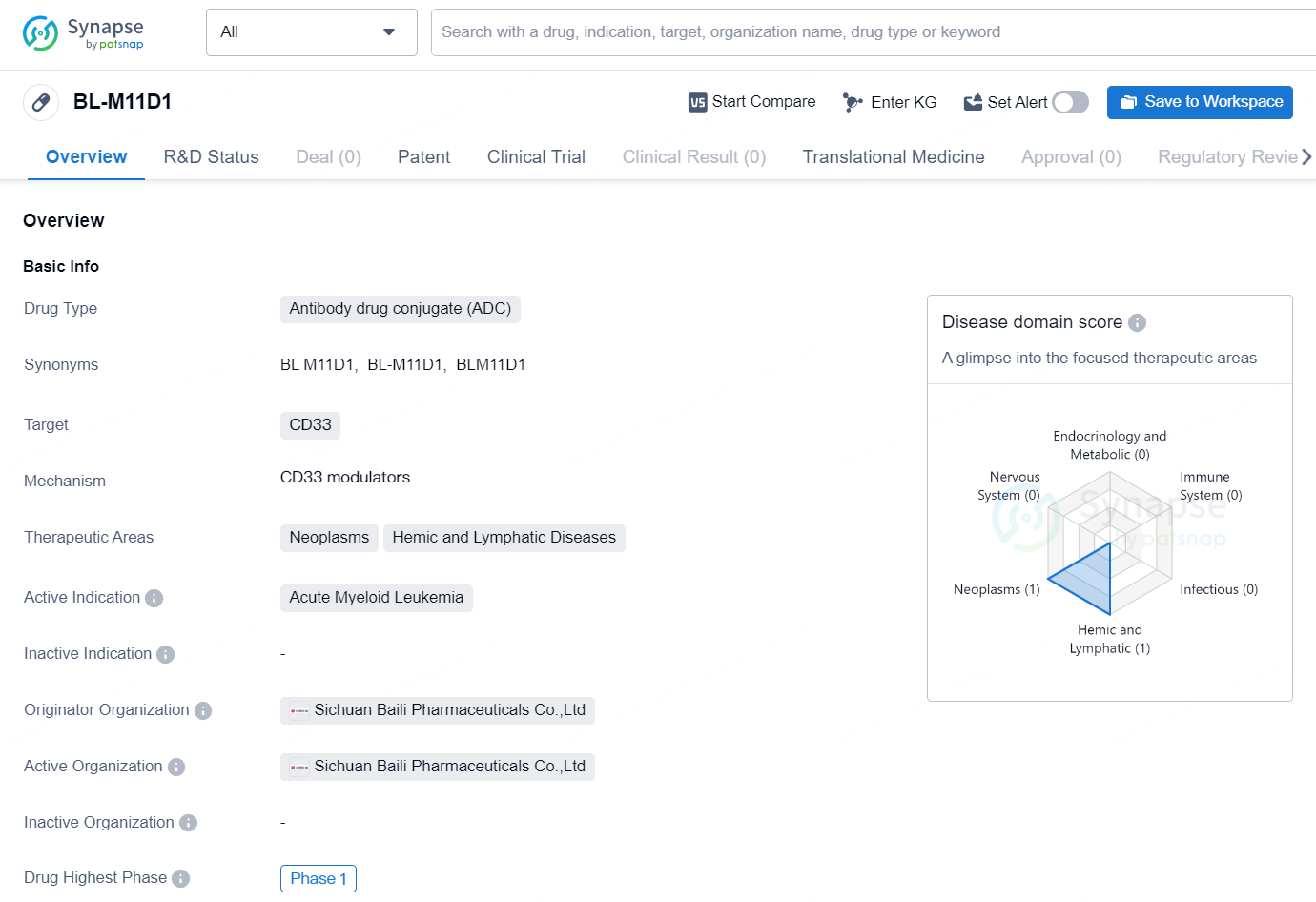
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The approval of this IND application represents a significant achievement for SystImmune as the firm advances its portfolio of innovative therapeutic candidates into the clinical trial phase. Dr. Jie D’Elia, the Chief Executive Officer of SystImmune, stated, “At SystImmune, our goal is to continue introducing therapies that can offer clinical advantages to patients. The launch of clinical development for BL-M11D1 underscores that commitment.”
“We are thrilled to have received the FDA Study-May-Proceed letter that allows us to begin our Phase 1 study with BL-M11D1. We believe this innovative, potentially best-in-class ADC could provide a crucial treatment option for patients with relapsed/refractory AML, and we are eager to start this study,” said Dr. Jonathan Cheng, Chief Medical Officer of SystImmune.
The company is in the process of developing BL-M11D1, an ADC that consists of a monoclonal antibody targeting CD33 paired with a linker-payload, which incorporates a topoisomerase I inhibitor and a stable enzyme-cleavable linker. BL-M11D1 functions by inducing antibody-dependent cellular cytotoxicity (ADCC) upon binding to CD33 on malignant cells. Furthermore, the attachment to CD33 leads to its internalization and subsequent release of the payload, ultimately leading to the destruction of the tumor cell.
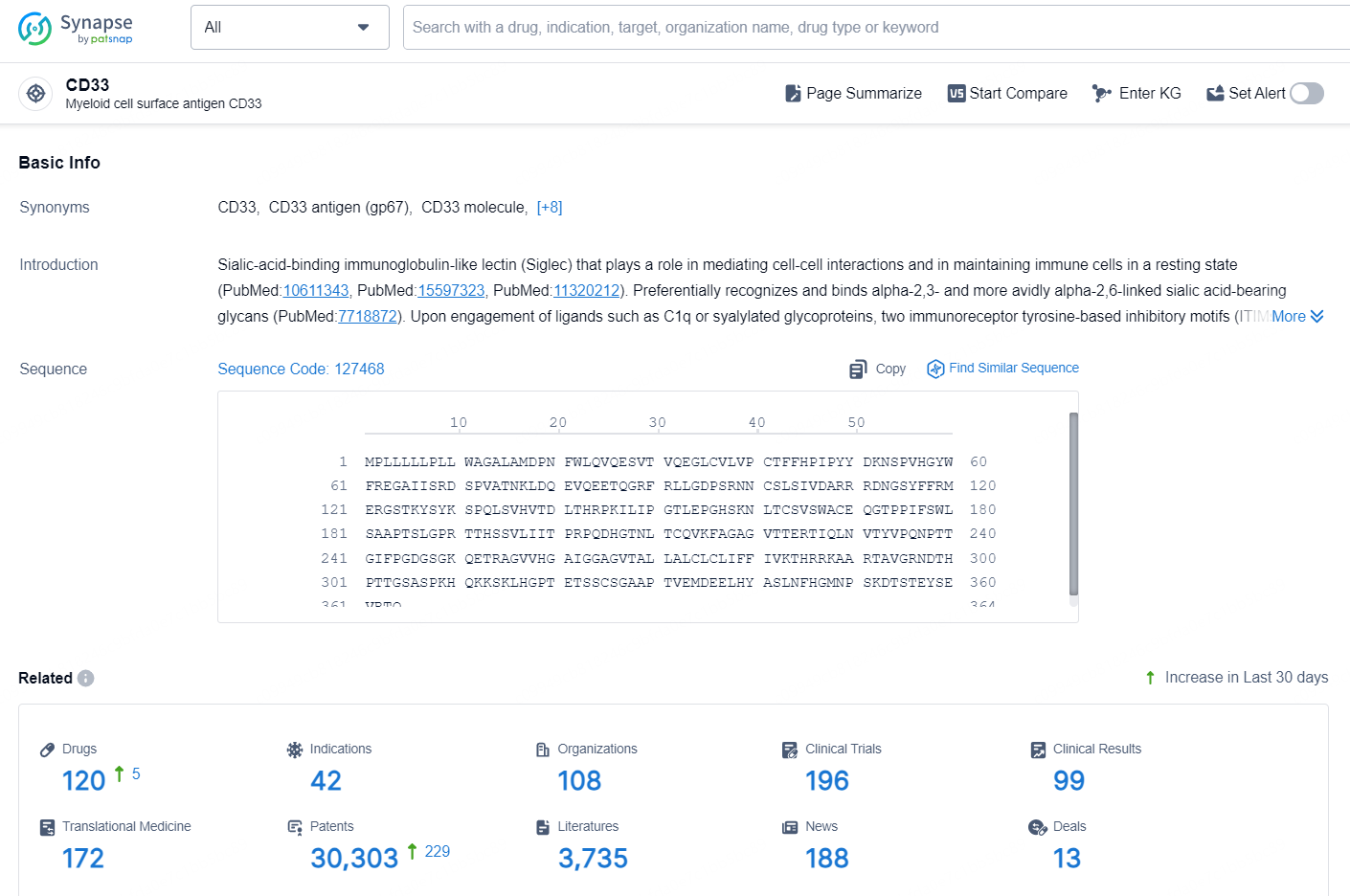
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 15, 2024, there are 120 investigational drugs for the CD33 targets, including 42 indications, 108 R&D institutions involved, with related clinical trials reaching 196, and as many as 30303 patents.
BL-M11D1 is an antibody drug conjugate (ADC) that targets CD33 and is being developed by Sichuan Baili Pharmaceuticals Co., Ltd. The drug is being investigated for its potential to treat neoplasms, hemic, and lymphatic diseases, with a specific focus on acute myeloid leukemia.