FDA Approves Pfizer’s HYMPAVZI™ for Hemophilia A and B in Adults and Teens Without Inhibitors
Pfizer Inc. (NYSE: PFE) reported that the U.S. Food and Drug Administration (FDA) has granted approval for HYMPAVZI™ (marstacimab-hncq) to be used as a standard prophylactic treatment aimed at preventing or decreasing the occurrence of bleeding episodes in adult and pediatric patients aged 12 and older who have hemophilia A (congenital factor VIII deficiency) without factor VIII (FVIII) inhibitors, or hemophilia B (congenital factor IX deficiency) without factor IX (FIX) inhibitors.
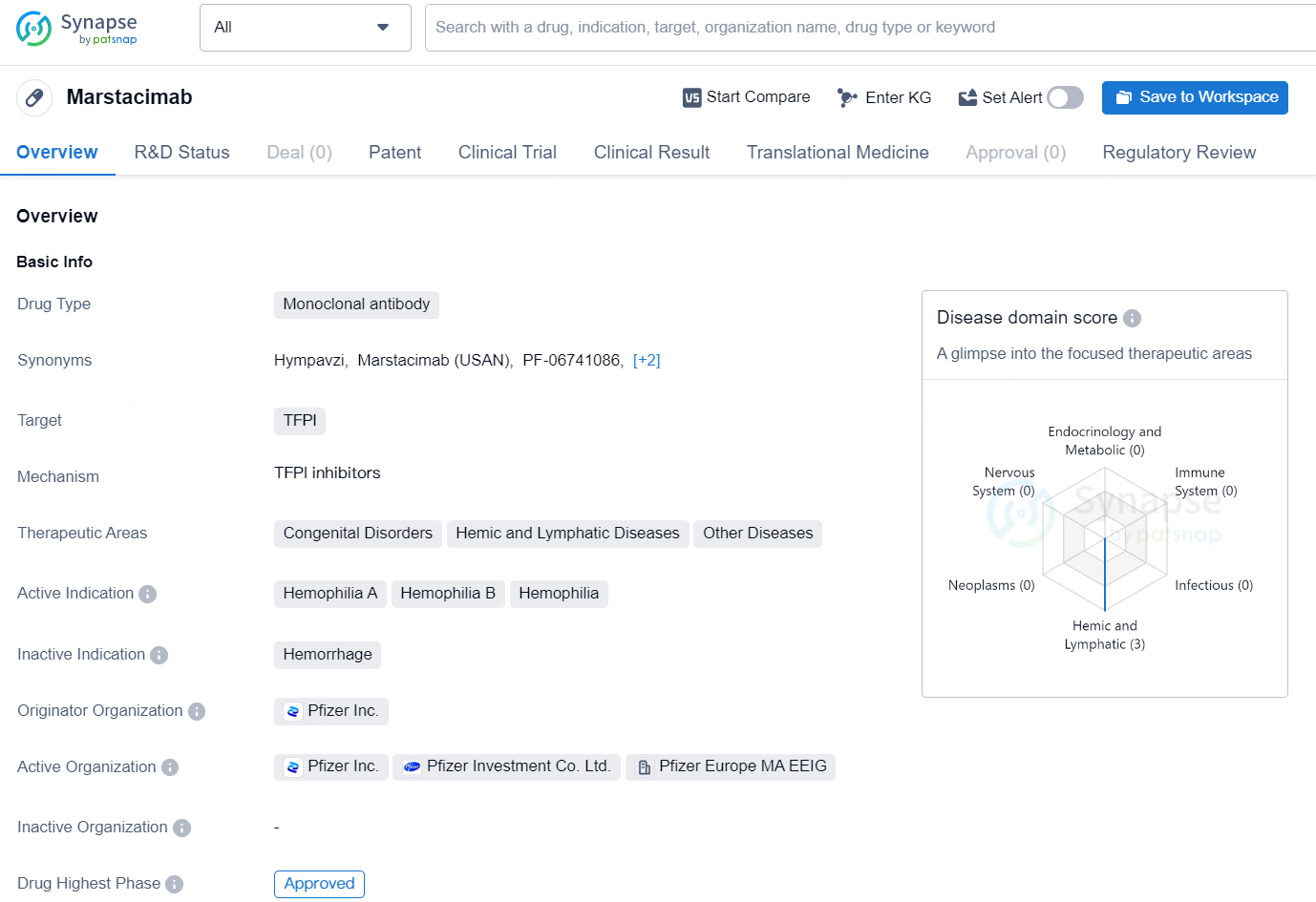
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
HYMPAVZI is the inaugural and sole anti-tissue factor pathway inhibitor (anti-TFPI) authorized in the U.S. for treating both hemophilia A and B. It represents the first hemophilia therapy in the U.S. to be delivered via a pre-filled, auto-injector pen. With a dosing schedule of once a week, HYMPAVZI provides a subcutaneous treatment option that requires minimal preparation for each administration.
“The approval of HYMPAVZI marks a significant step forward for individuals with hemophilia A or B who do not have inhibitors, offering an effective method for bleed prevention along with a manageable safety profile and simple weekly administrable subcutaneous option,” stated Suchitra S. Acharya, M.D., Director of the Hemostasis and Thrombosis Center at Northwell Health, and Program Head of Bleeding Disorders and Thrombosis at Cohen Children’s Medical Center. “This new therapy aims to alleviate the current treatment challenges faced by patients, particularly those who have depended on frequent and labor-intensive intravenous infusion protocols.”
Hemophilia refers to a group of rare genetic disorders characterized by deficiencies in clotting factors (FVIII in hemophilia A and FIX in hemophilia B), impacting over 800,000 individuals worldwide. Typically identified in early childhood, hemophilia impairs the body’s ability to form clots effectively, raising the likelihood of recurrent bleeding episodes in joints, potentially resulting in irreversible damage. Although there have been considerable advancements in hemophilia management recently, many affected individuals still endure bleeding incidents and rely on frequent intravenous infusions, often administered multiple times weekly.
“HYMPAVZI is Pfizer’s second hemophilia treatment to gain FDA approval this year, backing our ongoing dedication to enhancing care for hemophilia patients over the past four decades,” commented Aamir Malik, Chief U.S. Commercial Officer and Executive Vice President at Pfizer. “We are eager to introduce this innovative therapy and expand our offering to include three distinct categories of hemophilia treatments—anti-TFPI, gene therapy, and recombinant factor therapies—catering to the diverse needs of patients.”
The Phase 3 BASIS trial (NCT03938792) provided the evidence necessary for the FDA approval of HYMPAVZI for adults and adolescents with hemophilia A or B without inhibitors. The trial demonstrated that HYMPAVZI led to a 35% and 92% reduction in the annualized bleeding rate (ABR) for treated bleeds after a 12-month active treatment phase, compared to routine prophylaxis (RP) and on-demand (OD) treatment for patients with non-inhibitor hemophilia A or B. The safety profile of HYMPAVZI remained consistent with findings from Phase 1/2 studies, with the most commonly observed adverse effects (≥3% of patients) including injection site reactions, headaches, and itching.
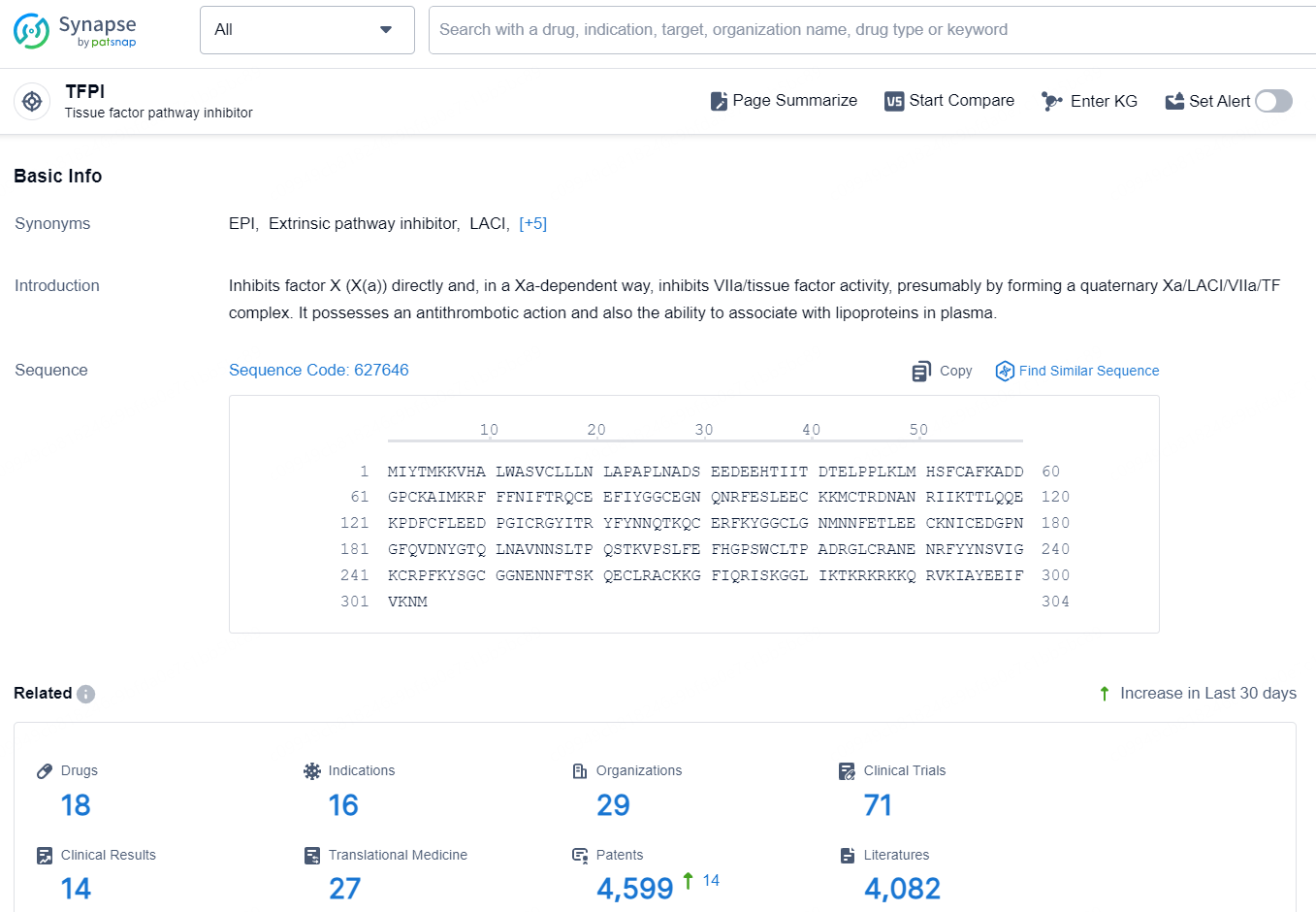
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 15, 2024, there are 18 investigational drugs for the TFPI targets, including 16 indications, 29 R&D institutions involved, with related clinical trials reaching 71, and as many as 4599 patents.
Marstacimab is a monoclonal antibody drug developed by Pfizer Inc., which targets TFPI (tissue factor pathway inhibitor). This drug falls under the therapeutic areas of Congenital Disorders, Hemic and Lymphatic Diseases, and Other Diseases, with active indications for Hemophilia A, Hemophilia B, and Hemophilia. The highest phase of development for Marstacimab globally is approved, while in China, it has reached the NDA/BLA (New Drug Application/Biologics License Application) phase. The drug was first approved in the United States in October 2024.