Sutro Biopharma showcases cutting-edge ADC technology and upcoming projects at research event
Sutro Biopharma, Inc. (Sutro or "the Company") (NASDAQ: STRO) is a clinical-stage oncology firm that specializes in innovative, site-specific antibody-drug conjugates (ADCs). the Company is conducting an investor webcast to showcase its unique cell-free platform utilized in the creation and advancement of next-generation ADCs. This includes a promising pipeline of distinct programs targeting a wide array of tumor types. The presentation will outline Sutro’s strategic methodology aimed at enhancing the therapeutic index of ADCs. Moreover, it will delve into the Company’s early-stage ADC development pipeline, which features STRO-004 (an ADC targeting tissue factor), dual-payload ADCs, and immunostimulatory ADCs (iADCs).
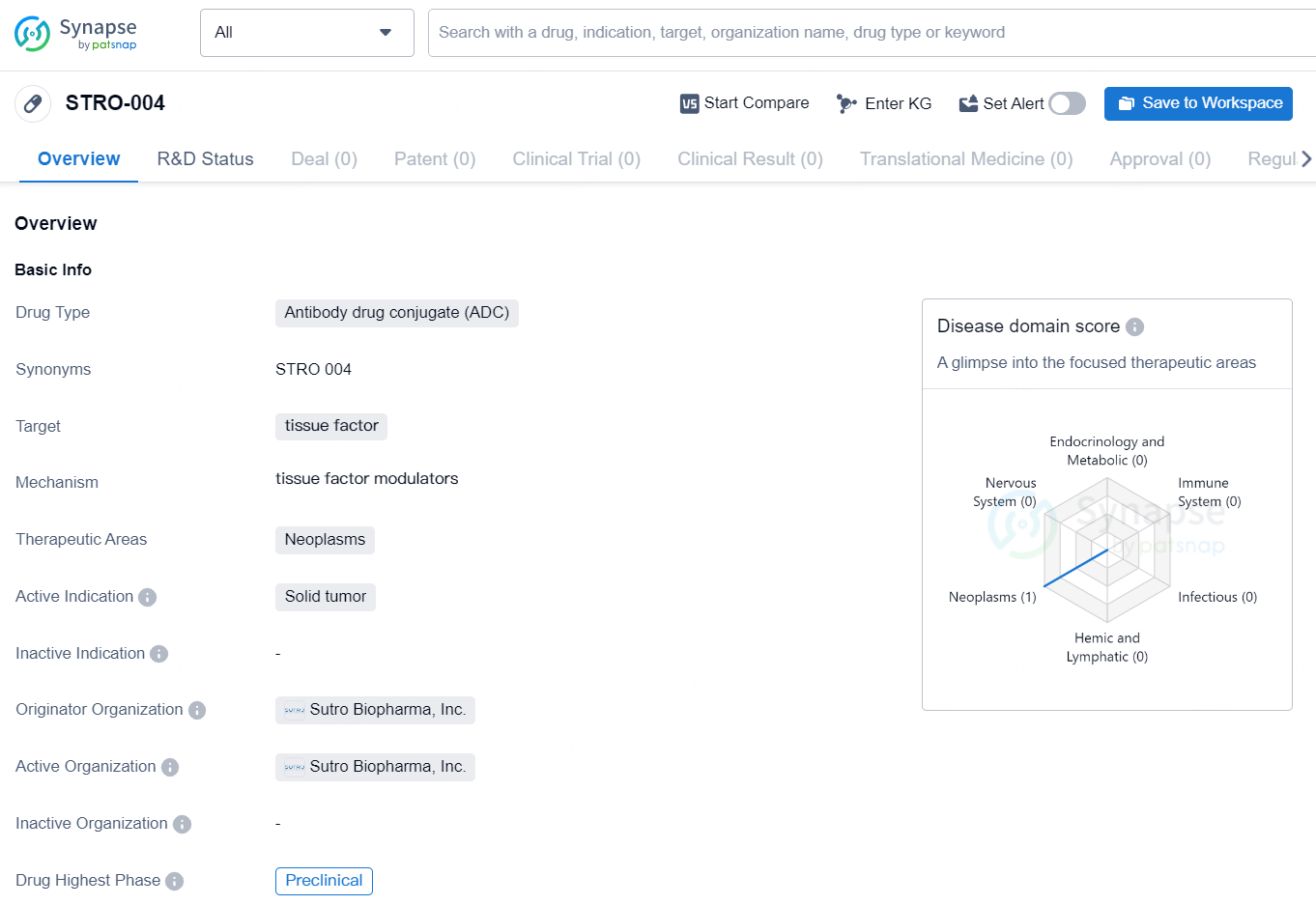
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Advancements in Next-Generation ADCs:
Improving ADC efficacy beyond the tumor environment: Sutro’s innovative cell-free platform allows for the incorporation of critical aspects of ADC design aimed at minimizing the toxicities seen with current-generation ADCs. These toxicities include interstitial lung disease, as well as skin, eye, liver, kidney issues, and thrombocytopenia. The platform encompasses groundbreaking technologies involving antibody design, payloads, linker systems, and conjugation chemistry, all of which have shown promise in preclinical studies for reducing toxic effects and enhancing pharmacokinetic profiles.
Improving ADC performance within tumor sites: Sutro’s cell-free platform supports three distinct strategies: 1) safely enhancing potency through a higher drug-antibody ratio (DAR) in exatecan ADCs; 2) utilizing dual-payload ADCs (ADC2) to combat tumor resistance; and 3) deploying next-generation immuno-oncology agents via immunostimulatory ADCs (iADCs), which merge immune stimulating effects with cytotoxic payloads.
Data Insights and Upcoming Pipeline Objectives:
The ADC STRO-004, designed to target tissue factor with a DAR8 exatecan payload and a site-specific linker, exhibited superior anti-tumor efficacy and reduced toxic side effects compared to a standard tissue factor ADC in preclinical tests. Sutro expects to submit an Investigational New Drug (IND) application for STRO-004 to the U.S. Food & Drug Administration by late 2025.
The dual-payload ADCs (ADC2) offer enhanced therapeutic effects over traditional ADCs by effectively tackling tumor resistance mechanisms, resulting in improved anti-tumor response and favorable attributes in preclinical evaluations.
Immunostimulatory ADCs (iADCs) introduce a novel action mechanism that connects innate and adaptive immune responses, offering a comprehensive protective effect within a single entity, and they demonstrate superior and sustained anti-tumor responses in preclinical models when compared to traditional ADCs or immune-stimulating antibody conjugates.
Sutro's exclusive and partnered preclinical ADC pipeline shows promise across multiple tumor types, and the company aims to file three IND applications in the next three years, including STRO-004 in the latter half of 2025. Additionally, Sutro plans to further develop an extensive pipeline of preclinical initiatives, enhancing opportunities for internal growth and potential collaborations.
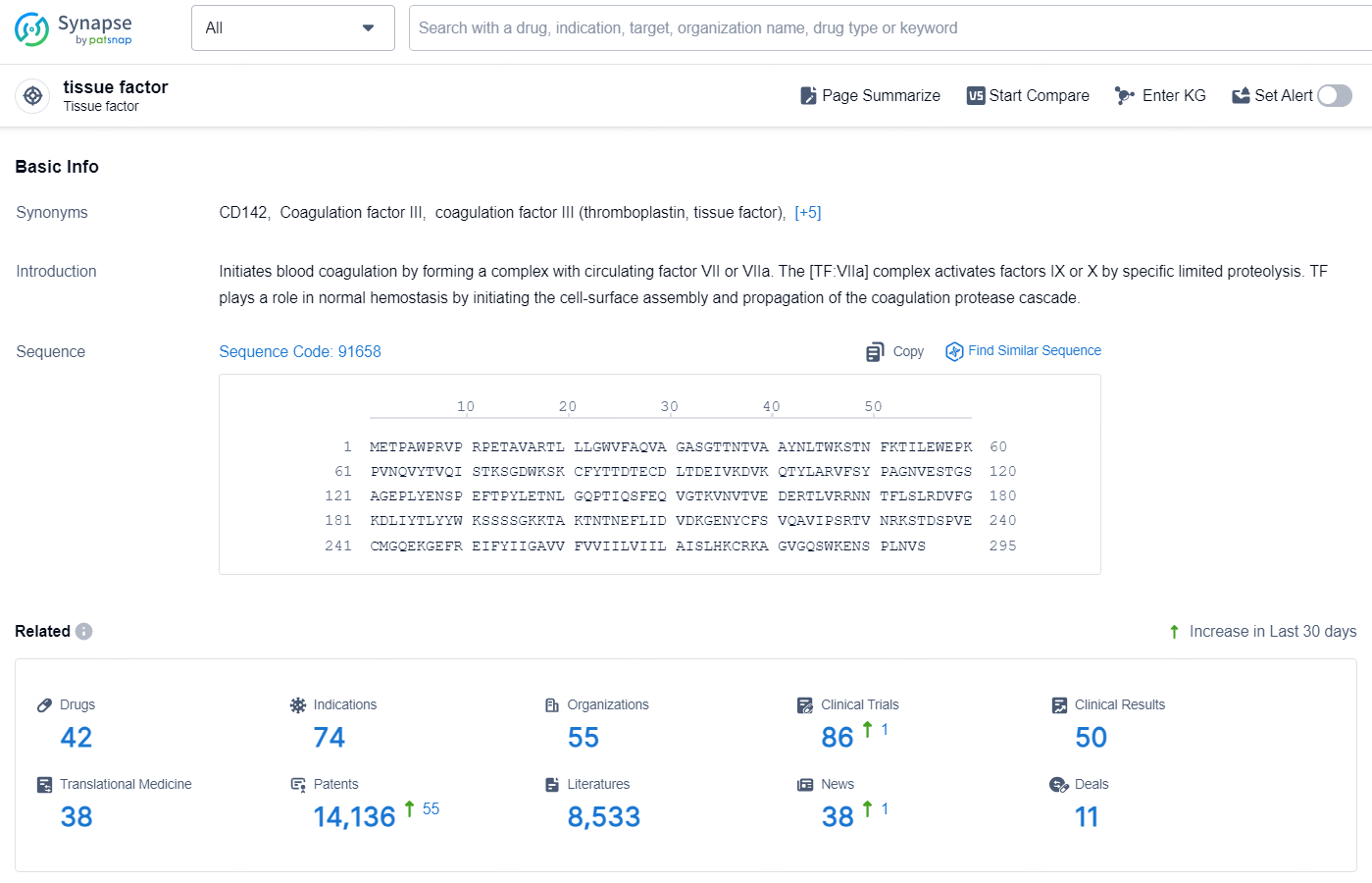
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 15, 2024, there are 42 investigational drug for the tissue factor target, including 74 indications, 55 R&D institutions involved, with related clinical trials reaching 86, and as many as 14136 patents.
STRO-004 is an antibody drug conjugate (ADC) targeting tissue factor. The therapeutic areas for this drug are neoplasms, and its active indication is solid tumor. Sutro Biopharma, Inc. is the originator organization of STRO-004, and it is currently in the preclinical phase, which is its highest phase globally.