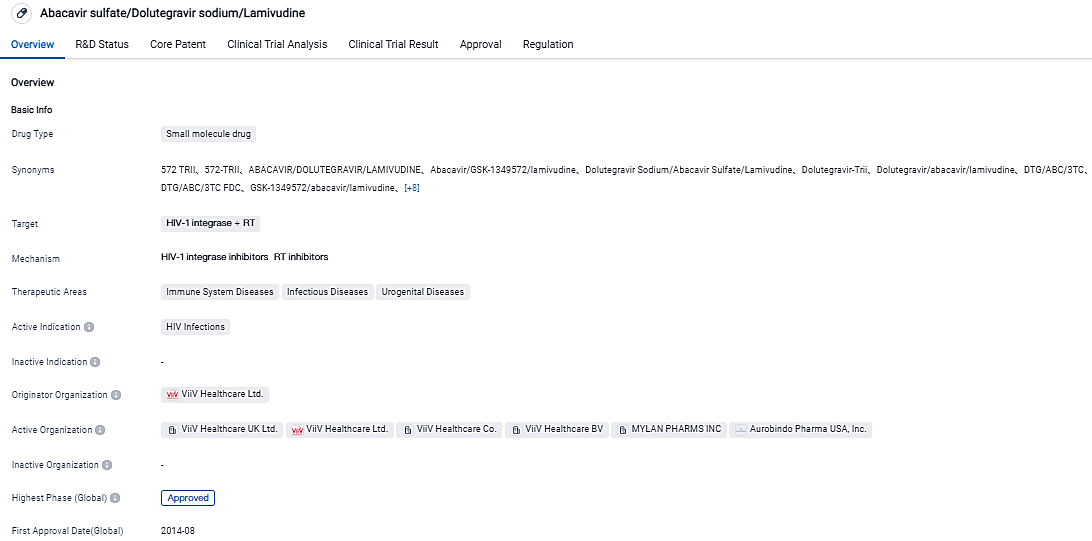
Viatris announced that the FDA has preliminarily approved the formulation of abacavir (ABC)/dolutigravir (DTG)/lamivudine (3TC) for children infected with HIV
The multinational healthcare firm, Viatris Inc., has shared news of the tentative approval from FDA)for an abacavir 60 mg/dolutegravir 5 mg/lamivudine 30 mg oral suspension tablet New Drug Application. This application is intended for the treatment of HIV-1 infection in young patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The World Health Organization endorses the use of abacavir/dolutegravir/lamivudine as the favoured initial treatment plan for pediatric patients. Treatment coverage for children and adolescents is reported lagging behind adults by the Joint United Nations Programme on HIV/AIDS.
Approximately 43 percent—or some 660,000— out of an estimated total of 1.5 million children living with HIV did not receive antiretroviral treatment in 2022. Consequently, children made up 13 percent of AIDS-related deaths in the same year, even though they represent merely about 4 percent of the HIV-positive population.
Strawberry-flavoured combination tablets for oral suspension have been developed as a fixed dosage. The treatment of pediatric HIV has been historically difficult, primarily because children need specially formulated medicines.
Rakesh Bamzai, the president of India, Emerging Asia & Access Markets at Viatris, implicated that Viatris has been providing increased access to premier HIV/AIDS treatment for over ten years. Bamzai added that they continually seek enhancements to current molecules to cater to patients efficiently.
Viatris has introduced new heat-resistant generic formulations, convenient packaging, pediatrics therapies, among other things. Their focus has been on enhancing access to ARVs for at-risk communities, such as children. The approval of this single-tablet treatment will ease the strain on young ones living with HIV.
The combination of abacavir/dolutegravir/lamivudine is approved to be used daily for the treatment in pediatric patients weighing between 6 and 25 kg afflicted with HIV-1. Hypersensitivity reaction to abacavir in the past and HLAB*5701-positive patients are contraindicated to consume the fixed-combination of abacavir/dolutegravir/lamivudine.
The preliminary approval allows the new child-friendly formulation to be submitted to regulatory authorities, manufactured, and distributed across 123 low- and middle-income countries under the license agreement.
Abacavir/dolutegravir/lamivudine is a fixed-dose combination containing two nucleoside reverse transcriptase inhibitors, which stop the reverse transcriptase and integrase enzymes, thus blocking virus replication. Each patient with HIV-1 should be examined for hepatitis B virus prior to or when beginning the fixed-dose combination of abacavir/dolutegravir/lamivudine, due to reports of severe acute exacerbations of HBV in co-infected patients who have stopped the medication.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
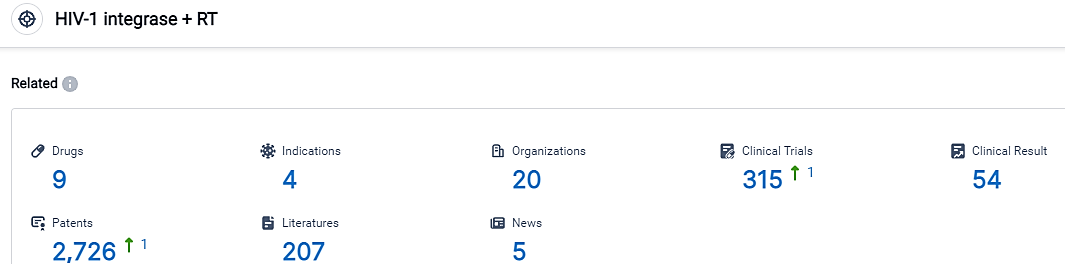 According to the data provided by the Synapse Database, As of September 6, 2023, there are 9 investigational drugs for the HIV-1 intergrase and RT target, including 4 applicable indications, 20 R&D institutions involved, with related clinical trials reaching 315, and as many as 2726 patents.
According to the data provided by the Synapse Database, As of September 6, 2023, there are 9 investigational drugs for the HIV-1 intergrase and RT target, including 4 applicable indications, 20 R&D institutions involved, with related clinical trials reaching 315, and as many as 2726 patents.
The use of abacavir/dolutegravir/lamivudine aids the goal of sustainable provision of ARV treatment to approximately 30 million individuals, inclusive of over 2 million kids afflicted with HIV/AIDS, within the timeframe of 2022 to the conclusion of 2025.