iTeos and GSK Initiate Phase 3 GALAXIES Lung-301 Trial for Advanced Lung Cancer Treatment
iTeos Therapeutics, Inc., a biopharmaceutical firm in the clinical trial phase innovating a novel class of immuno-oncology treatments, along with its collaborator GSK, has launched the inaugural worldwide Phase 3 registration trial. This study examines the efficacy of the combination therapy belrestotug and dostarlimab against a placebo coupled with pembrolizumab in individuals with previously untreated, inoperable, locally advanced, or metastatic non-small cell lung cancer that expresses PD-L1.
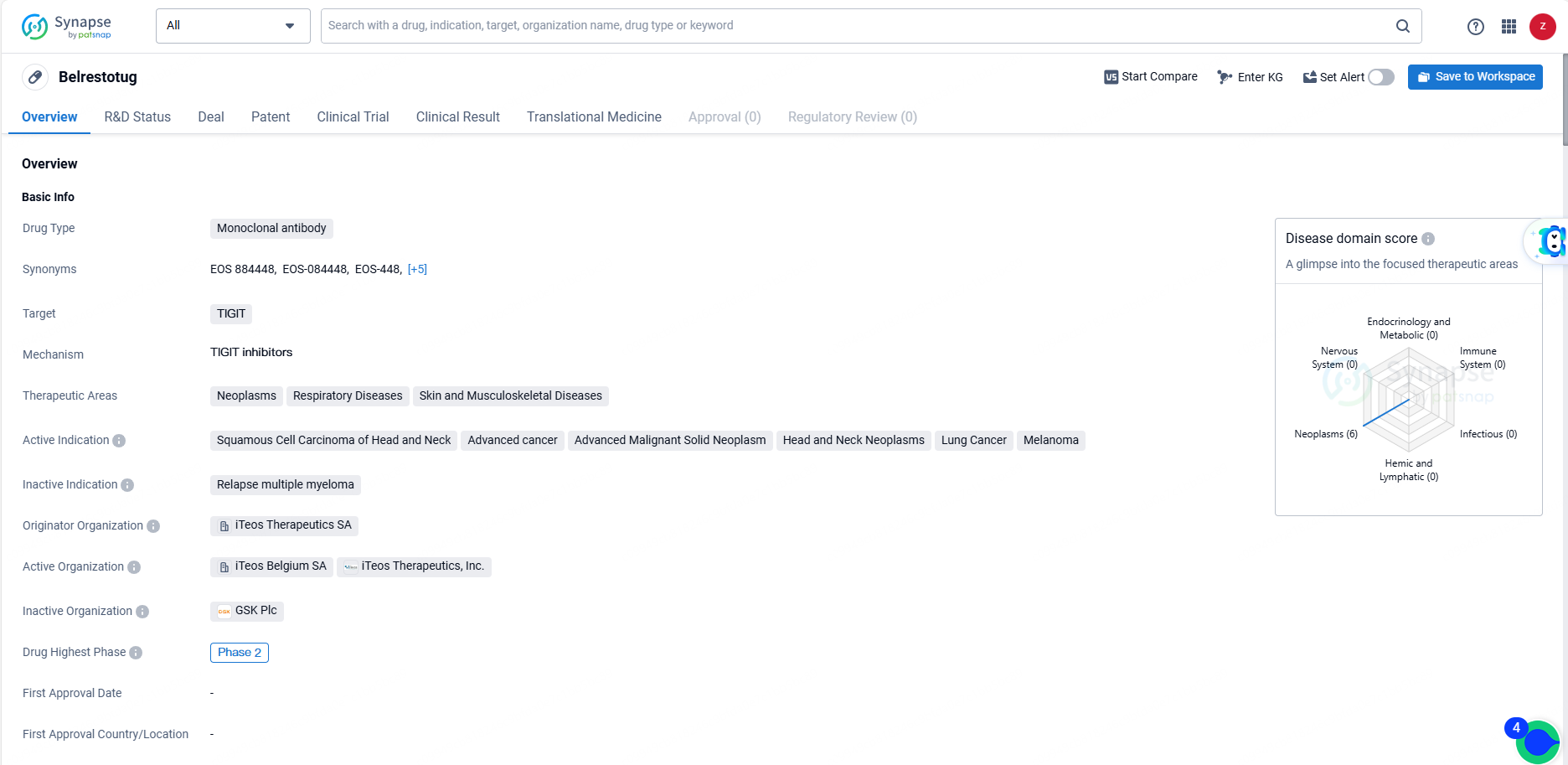
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Initiating the first Phase 3 study for belrestotug marks a significant milestone in our mission to establish a premier oncology company," said Michel Detheux, Ph.D., president and CEO of iTeos. "Around 70% of patients with first-line PD-L1 high non-small cell lung cancer (NSCLC) depend on a regimen free from chemotherapy. We believe that the combination of belrestotug and dostarlimab has the potential to enhance treatment protocols in this context, setting new standards."
Dr. Detheux further explained, "The high-quality doublet demonstrated greater efficacy than the pre-defined criteria for clinical activity in an interim analysis of the Phase 2 GALAXIES Lung-201 study. This supports our decision to commence the Phase 3 program for this patient group, which is integral to our overarching strategy and a critical step toward building our franchise."
The randomized, double-blind, placebo-controlled, multicenter trial will involve approximately 1,000 patients with previously untreated, unresectable, locally advanced or metastatic PD-L1 selected NSCLC across North America, South America, Europe, and Asia. The trial's primary endpoints are progression-free survival and overall survival. In the GALAXIES Lung-301 trial, patients will be randomly assigned in a 1:1 ratio to receive either intravenous belrestotug + dostarlimab doublet or placebo + pembrolizumab.
In May 2024, iTeos reported an interim analysis of the Phase 2 GALAXIES Lung-201 study, revealing that the belrestotug + dostarlimab combination surpassed predefined efficacy criteria for clinically meaningful activity and exhibited a safety profile consistent with the TIGIT: PD-1 class. Significant tumor reduction was noted with every dose of the belrestotug + dostarlimab combination compared to dostarlimab monotherapy.
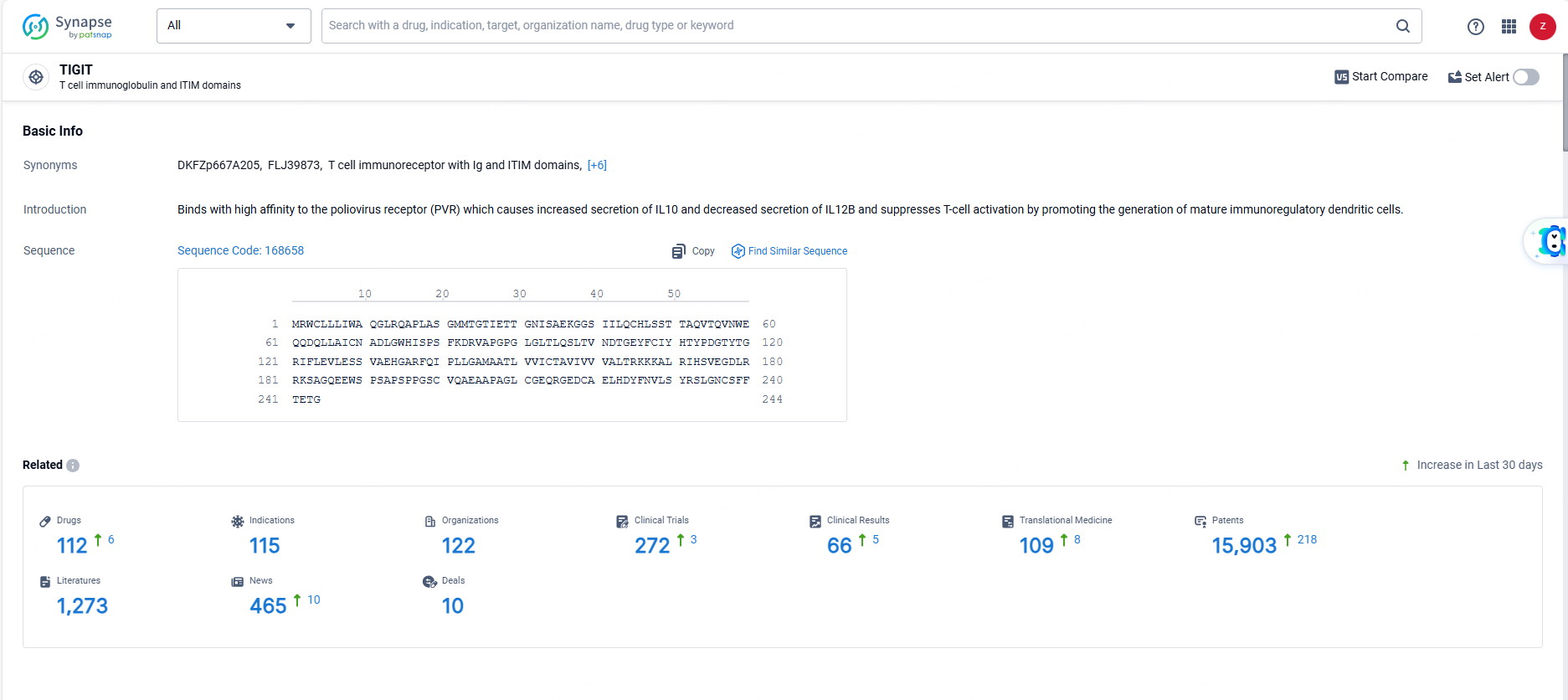
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 21, 2024, there are 112 investigational drugs for the TIGIT target, including 115 indications, 122 R&D institutions involved, with related clinical trials reaching 272, and as many as 15903 patents.
Belrestotug as a monoclonal antibody targeting TIGIT represents an important advancement in the field of biomedicine, particularly in the treatment of various cancers and related conditions. As the drug progresses through clinical trials, further data will be needed to fully assess its safety and efficacy, but the initial indications suggest that it holds promise as a potential new treatment option for patients with advanced malignancies.