The combination regimen of amitabacizumab demonstrated significant effects in the Phase 3 MARIPSA-2 trial
Johnson's Janssen Pharmaceutical Companies have disclosed encouraging preliminary findings from the tri-modality Phase 3 MARIPOSA-2 trial. This research assessed RYBREVANT®(amivantamab-vmjw), a dual-specificity antibody that targets the epidermal growth factor receptor and the mesenchymal-epithelial transition. It was administered either with or without lazertinib, an orally taken, third-generation EGFR tyrosine kinase inhibitor, in conjunction with chemotherapy as opposed to merely chemotherapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The MARIPOSA-2 trial enrolled participants who had locally progressed or metastatic EGFR exon 19 deletions or L858R substitution NSCLC following disease progression post osimertinib treatment. The study fulfilled its dual primary outcome, revealing significant and clinically meaningful PFS improvement as compared to standalone chemotherapy in both experimental treatment groups. RYBREVANT®'s addition to chemotherapy showed no new safety signals.
The results are planned to be submitted by Janssen for discussion at upcoming scientific conferences, including information on secondary outcomes like OS, objective response, response duration, and intracranial PFS.
Dr. Peter Lebowitz, M.D., Ph.D., the Global Therapeutic Area Head of Oncology at Janssen Research & Development, LLC stated, "MARIPOSA-2 is the inaugural Phase 3 study data for RYBREVANT-based treatments in the wider EGFR-mutated NSCLC population. The study leverages the substantial advancement of RYBREVANT, the pioneer bispecific antibody that targets two principle oncogenic driver pathways, delivering clinically meaningful outcomes that could transform the treatment approach."
MARIPOSA-2 is a randomized, open-label Phase 3 assessment of the safety and effectiveness of two RYBREVANT® and chemotherapy regimens. Patients who had locally advanced or metastatic EGFR ex19del or L858R NSCLC, and had disease progression on or post osimertinib treatment were randomized to receive either RYBREVANT® plus chemotherapy, RYBREVANT® plus chemotherapy with lazertinib, or standalone chemotherapy.
The dual primary outcome was employed to examine the PFS as evaluated by the blinded independent central review for each experimental arm versus standalone chemotherapy. Secondary outcomes included objective response as evaluated by BICR, OS, DoR, time to subsequent treatment, PFS post first subsequent treatment, and intracranial PFS.
As brain metastases can result in substantial illnesses and poor patient outcomes, this research design aspect delivers critical information in a high need area. A total of 657 patients with locally advanced or metastatic EGFR ex19del or L858R were enrolled in the study, all having disease progression on or after osimertinib.
In the key Phase 3 assessment, a combination of RYBREVANT® and lazertinib is also being assessed for first-line treatment in patients with EGFR-mutated NSCLC. MARIPOSA evaluates the combination therapy of RYBREVANT® and lazertinib directly against osimertinib, with an additional lazertinib arm to evaluate component contribution.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
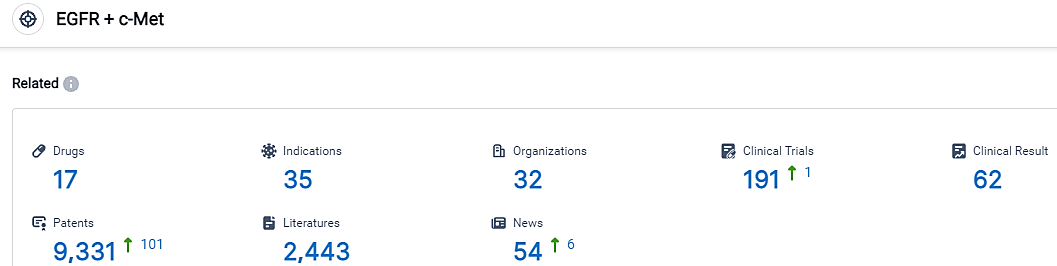
According to the data provided by the Synapse Database, As of September 8, 2023, there are 17 investigational drugs for the EGFR and c-Met target, including 35 applicable indications,32 R&D institutions involved, with related clinical trials reaching 191,and as many as 9331 patents.
RYBREVANT® (amivantamab-vmjw), a human-made bispecific antibody engaging EGFR and c-MET and acting by steering immune cells, was fast-tracked for approval by the FDA in May 2021. It's intended for the therapeutic management of adult individuals struggling with locally evolved or metastasized NSCLC possessing EGFR exon 20 insertion flaws, identifiable by an FDA-permitted assay, and whose ailment has progressed during or subsequent to platinum-intensive chemotherapy. RYBREVANT® also gained validation from European health agencies and others worldwide.