FDA Grants Fast-Track Approval to Novartis' Fabhalta for Lowering Proteinuria in IgAN
Novartis revealed that the U.S. Food and Drug Administration (FDA) has given accelerated approval for Fabhalta (iptacopan), an innovative complement inhibitor aimed at lowering proteinuria in adults suffering from primary immunoglobulin A nephropathy who are at risk of swift disease advancement. This condition is commonly identified by a urine protein-to-creatinine ratio (UPCR) of 1.5 g/g or higher.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 Fabhalta is designed to specifically target the alternative complement pathway in the immune system. When excessively activated in the kidneys, this complement system is believed to play a role in the development of IgAN.
Fabhalta is designed to specifically target the alternative complement pathway in the immune system. When excessively activated in the kidneys, this complement system is believed to play a role in the development of IgAN.
The indication for Fabhalta has received accelerated approval based on a pre-specified interim analysis of the Phase III APPLAUSE-IgAN study, which looked at the reduction in proteinuria at 9 months compared to a placebo. It has not yet been determined whether Fabhalta slows the decline of kidney function in patients with IgAN.
Continued approval may depend on confirming and describing the clinical benefits in the ongoing Phase III APPLAUSE-IgAN study, which is assessing whether Fabhalta impedes disease progression by measuring the decline in estimated glomerular filtration rate over 24 months. The eGFR data, expected upon study completion in 2025, are intended to support traditional FDA approval.
IgAN is a progressive, rare condition where the immune system attacks the kidneys, often leading to glomerular inflammation and proteinuria. Annually, about 25 people per million globally are newly diagnosed with IgAN. The progression and response to treatment vary for each individual.
Despite receiving the current standard of care, up to 50% of IgAN patients with persistent proteinuria advance to kidney failure within 10 to 20 years after diagnosis. These patients often need ongoing dialysis and/or kidney transplantation. Effective, targeted therapies with different mechanisms of action can aid physicians in selecting the most suitable treatment for patients.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
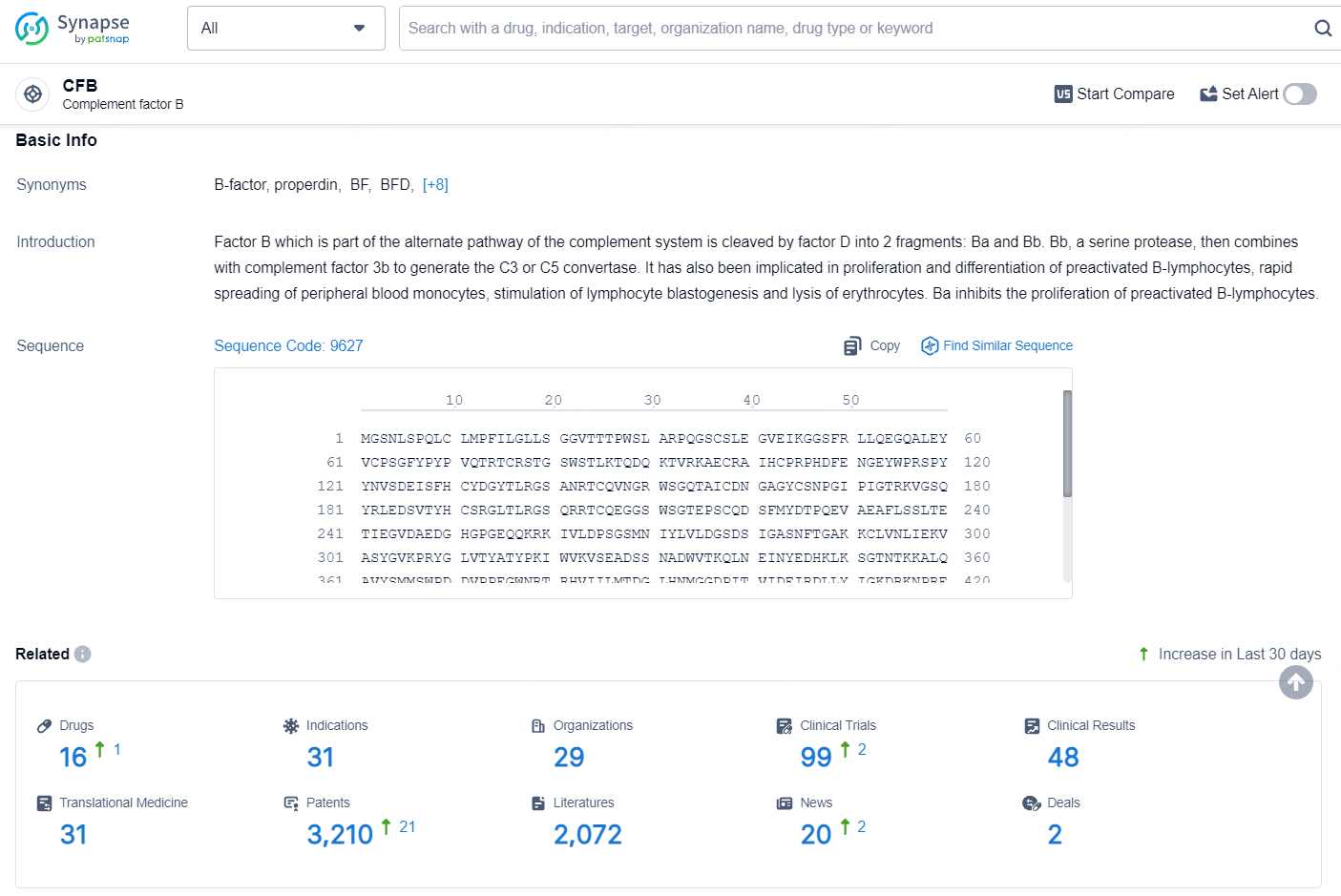
According to the data provided by the Synapse Database, As of August 12, 2024, there are 16 investigational drugs for the CFB target, including 31 indications, 29 R&D institutions involved, with related clinical trials reaching 99, and as many as 3210 patents.
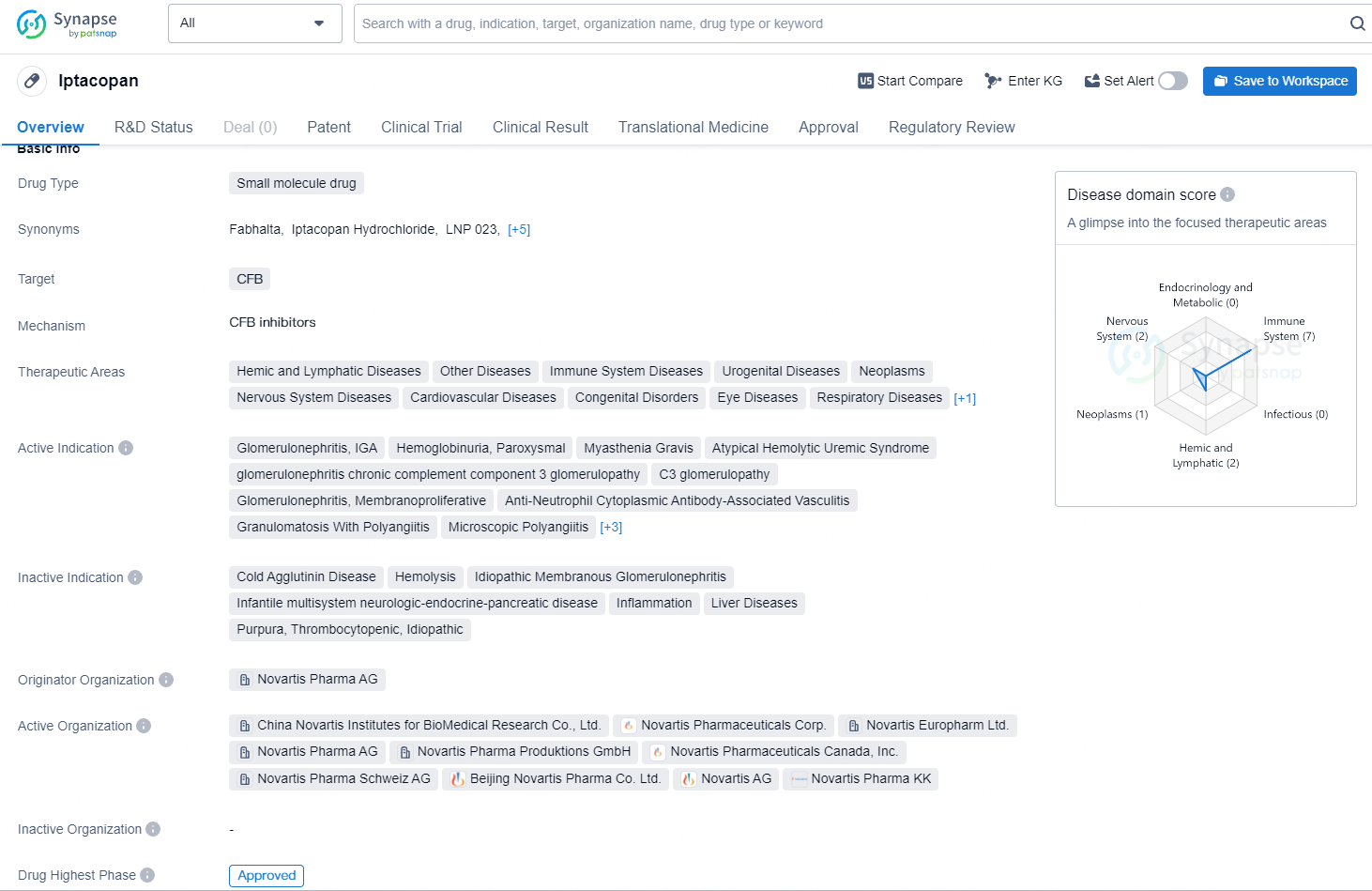
Iptacopan targets the complement factor B (CFB) and is indicated for various therapeutic areas including Hemic and Lymphatic Diseases, Immune System Diseases, Neoplasms, Cardiovascular Diseases, and others. Iptacopan represents a significant advancement in the pharmaceutical industry, holding promise for addressing a wide range of diseases across diverse therapeutic areas. Its approval and regulatory designations position it as a potential game-changer in the treatment of rare and pediatric conditions, offering hope for improved outcomes for patients globally.