Invenra Highlights Exelixis' Phase 1 Trial for XB010 in Advanced Tumor Patients
Invenra's collaborator, Exelixis, Inc., has reported the commencement of the dose-escalation phase for the first-in-human phase 1 clinical study of XB010 in individuals with locally advanced or metastatic solid tumors.
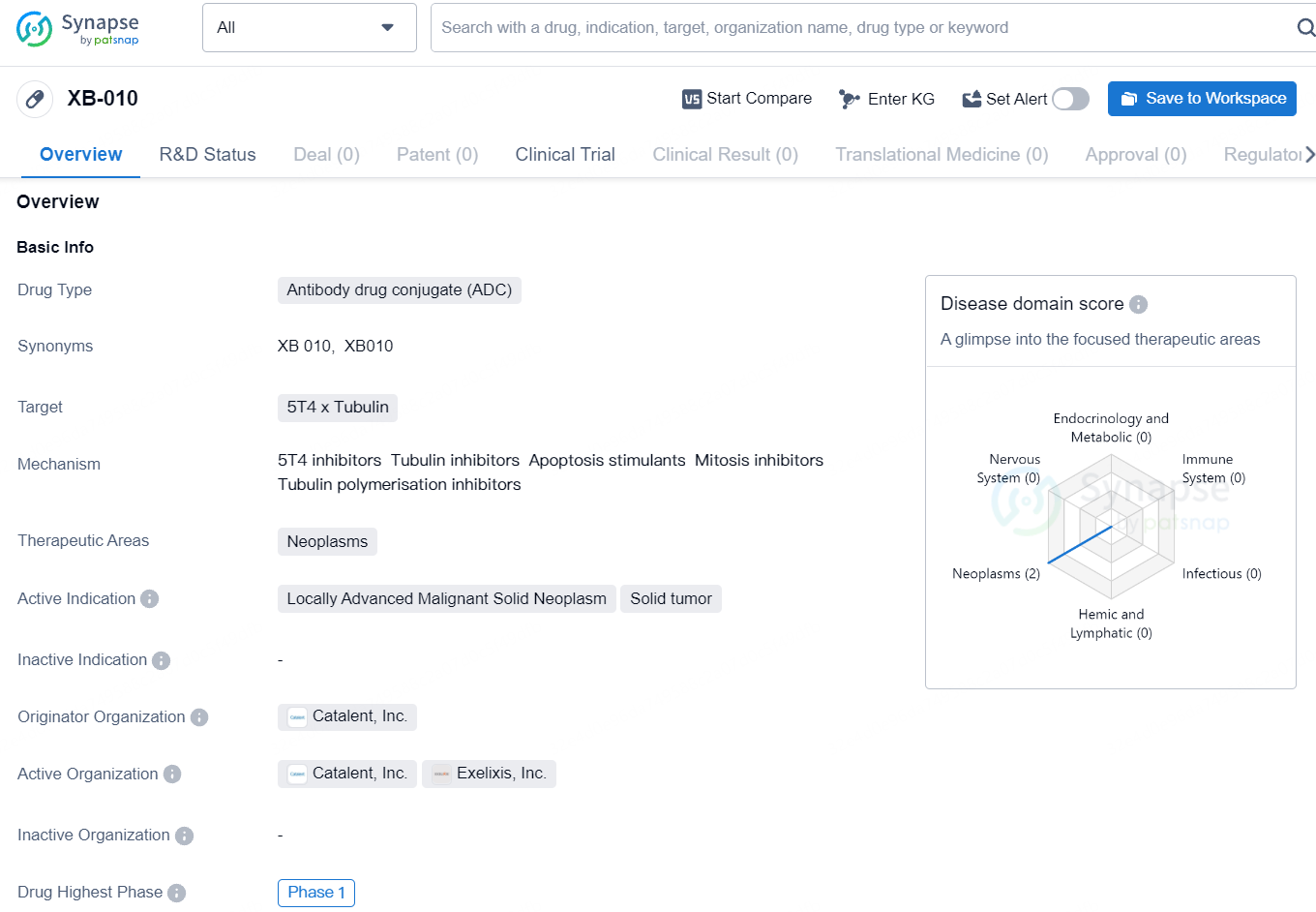
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
XB010 is an antibody-drug conjugate that combines a monomethyl auristatin E payload with a monoclonal antibody targeting the 5T4 tumor antigen. It represents the first tailor-made ADC developed through Exelixis’ biotherapeutics collaboration network. The creation of XB010 utilized Catalent’s SMARTag platform for site-specific bioconjugation, and the 5T4-targeting monoclonal antibody was discovered in partnership with Invenra.
Exelixis has also announced the commencement of the dose-escalation phase of their phase 1 global, open-label study. This phase will evaluate XB010 both as a monotherapy and in combination with pembrolizumab to gather data for the next cohort-expansion phase. The expansion cohorts are aimed at further evaluating the tolerability and therapeutic activity of the monotherapy and combination in selected indications.
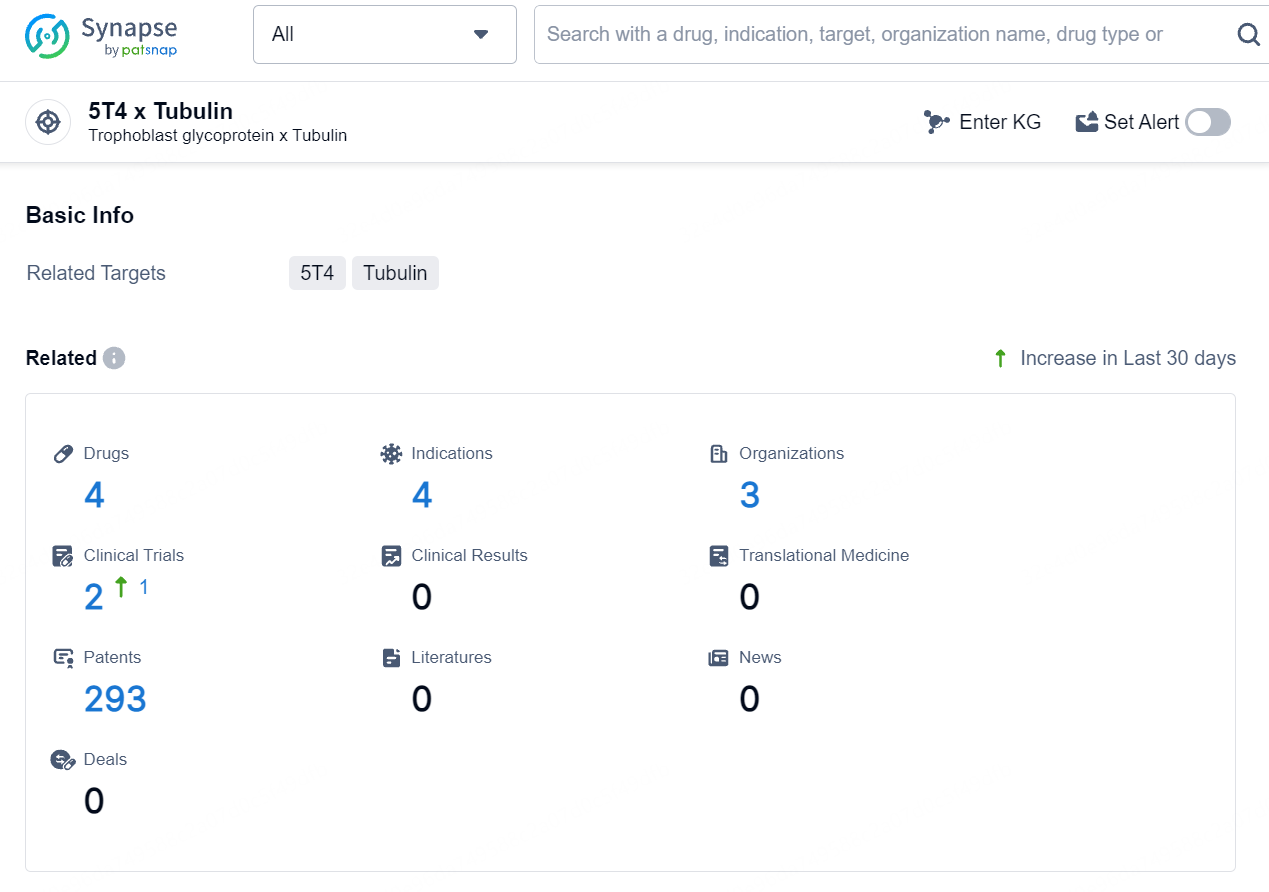
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 12, 2024, there are 4 investigational drugs for the 5T4 and Tubulin target, including 4 indications, 3 R&D institutions involved, with related clinical trials reaching 2, and as many as 293 patents.
As XB-010 is still in the preclinical phase, it has not yet advanced to clinical trials in humans. However, preclinical studies are crucial for evaluating the safety and efficacy of the drug, as well as understanding its pharmacokinetics and pharmacodynamics. Once preclinical development is complete, XB-010 will move into clinical trials to further assess its potential as a treatment for solid tumors in neoplasms.