FDA Grants Priority Review to Sarclisa for Newly Diagnosed Multiple Myeloma Patients Ineligible for Transplant
The supplemental Biologics License Application for the experimental usage of Sarclisa (isatuximab) combined with bortezomib, lenalidomide, and dexamethasone has been accepted for Priority Review by the U.S. Food and Drug Administration. This is intended for treating patients with newly diagnosed multiple myeloma who are not eligible for transplantation.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
If given the green light, Sarclisa would be the first anti-CD38 therapy combined with the standard VRd regimen for newly diagnosed patients ineligible for transplants, marking Sarclisa's third approved use in multiple myeloma. The FDA decision is anticipated by September 27, 2024, and a submission is also being reviewed in the European Union.
"Even with recent progress in multiple myeloma treatments, there's still a pressing need for new first-line therapies, especially for patients not eligible for transplants who often face poor disease outcomes. The acceptance of our filings and the FDA’s Priority Review designation bolster our belief in Sarclisa as a potentially superior treatment option and are vital steps forward in addressing this challenging cancer," commented Dietmar Berger, M.D., Ph.D., Chief Medical Officer and Global Head of Development at Sanofi.
The sBLA and EU submission are grounded in positive data from the IMROZ phase 3 trial, which assessed Sarclisa combined with VRd. In December 2023, the study achieved its primary endpoint during an interim efficacy analysis, showing a statistically significant increase in progression-free survival for Sarclisa with VRd compared to VRd alone in transplant-ineligible patients with newly diagnosed multiple myeloma. The safety and tolerability profile of Sarclisa in this study aligned with its known safety characteristics in combination with VRd.
Sarclisa is a monoclonal antibody targeting a specific epitope on the CD38 receptor on multiple myeloma cells and triggers distinct antitumor activity. It operates through various mechanisms, including programmed tumor cell death and immunomodulatory activities. CD38 is predominantly expressed on the surface of myeloma cells, making it a viable target for antibody-based treatments like Sarclisa.
Ongoing phase 3 trials continue to evaluate Sarclisa in combination with current standard therapies across the multiple myeloma treatment spectrum. It is also being explored for treating other hematologic cancers, although its safety and efficacy for these indications have not been evaluated by any regulatory authorities outside its approved use.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
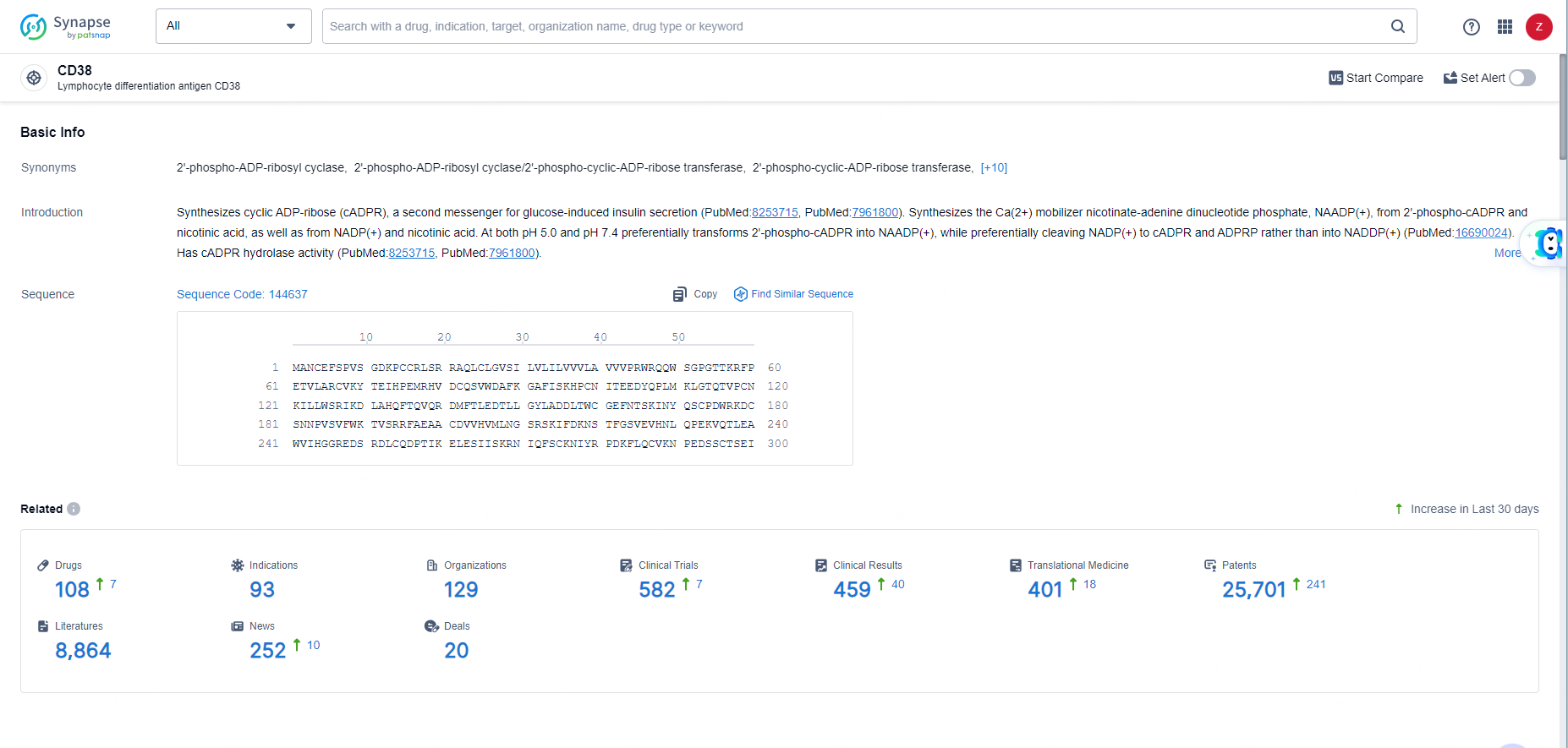 According to the data provided by the Synapse Database, As of May 31, 2024, there are 108 investigational drugs for the CD38 target, including93 indications, 129 R&D institutions involved, with related clinical trials reaching 582, and as many as 25701 patents.
According to the data provided by the Synapse Database, As of May 31, 2024, there are 108 investigational drugs for the CD38 target, including93 indications, 129 R&D institutions involved, with related clinical trials reaching 582, and as many as 25701 patents.
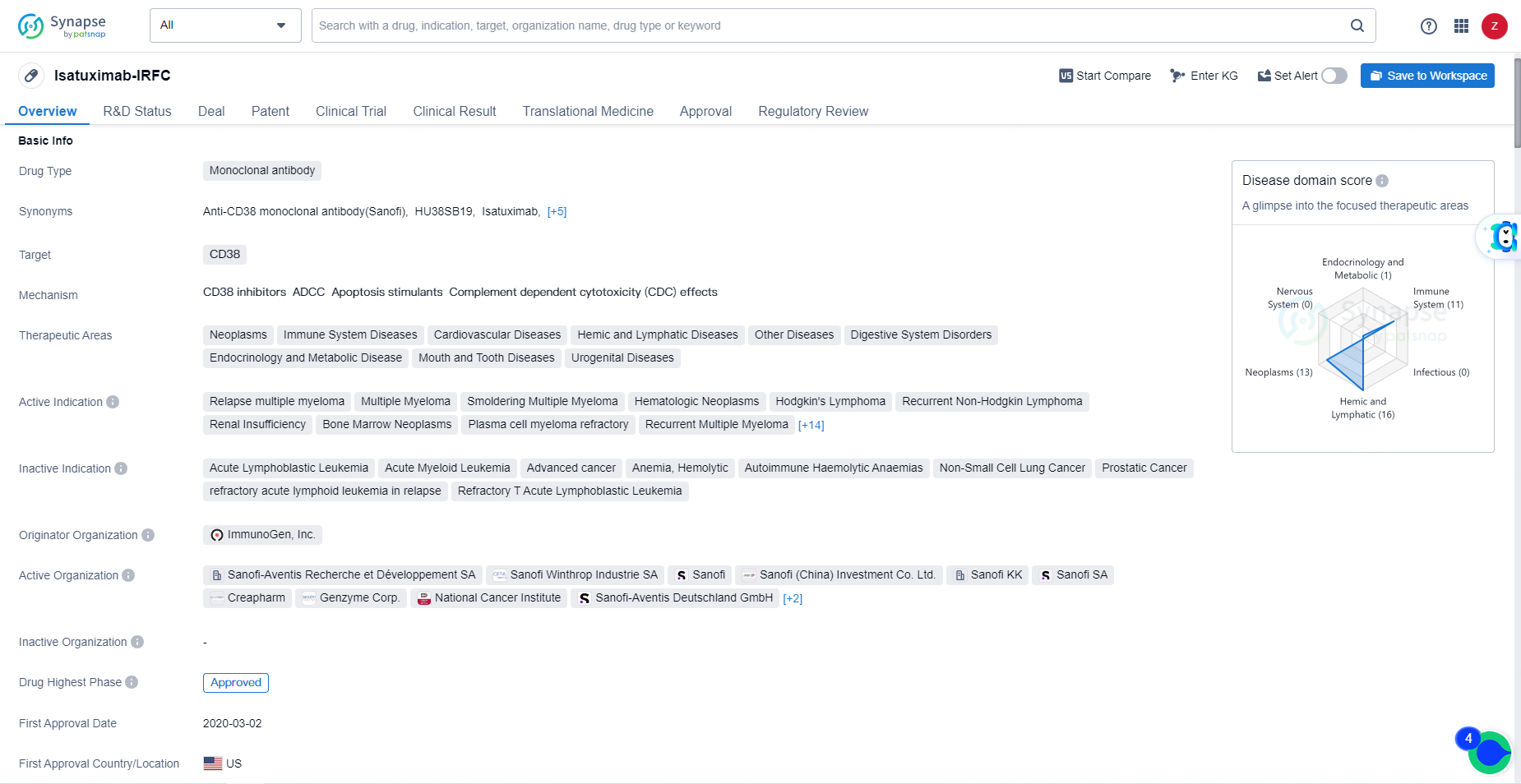
Isatuximab-IRFC is a monoclonal antibody drug with a broad range of therapeutic indications and has shown promising results in clinical trials. Its approval and regulatory status reflect its potential to address unmet medical needs in the treatment of various hematologic neoplasms and related conditions.