FDA Greenlights Initial Therapy for Niemann-Pick Type C Disease
The U.S. Food and Drug Administration has greenlit Miplyffa (arimoclomol), an oral drug designed for managing Niemann-Pick disease type C (NPC). When used along with the enzyme inhibitor miglustat, Miplyffa is sanctioned to address the neurological symptoms in NPC patients aged 2 and older. This marks the FDA's first approval of a medication targeting NPC.
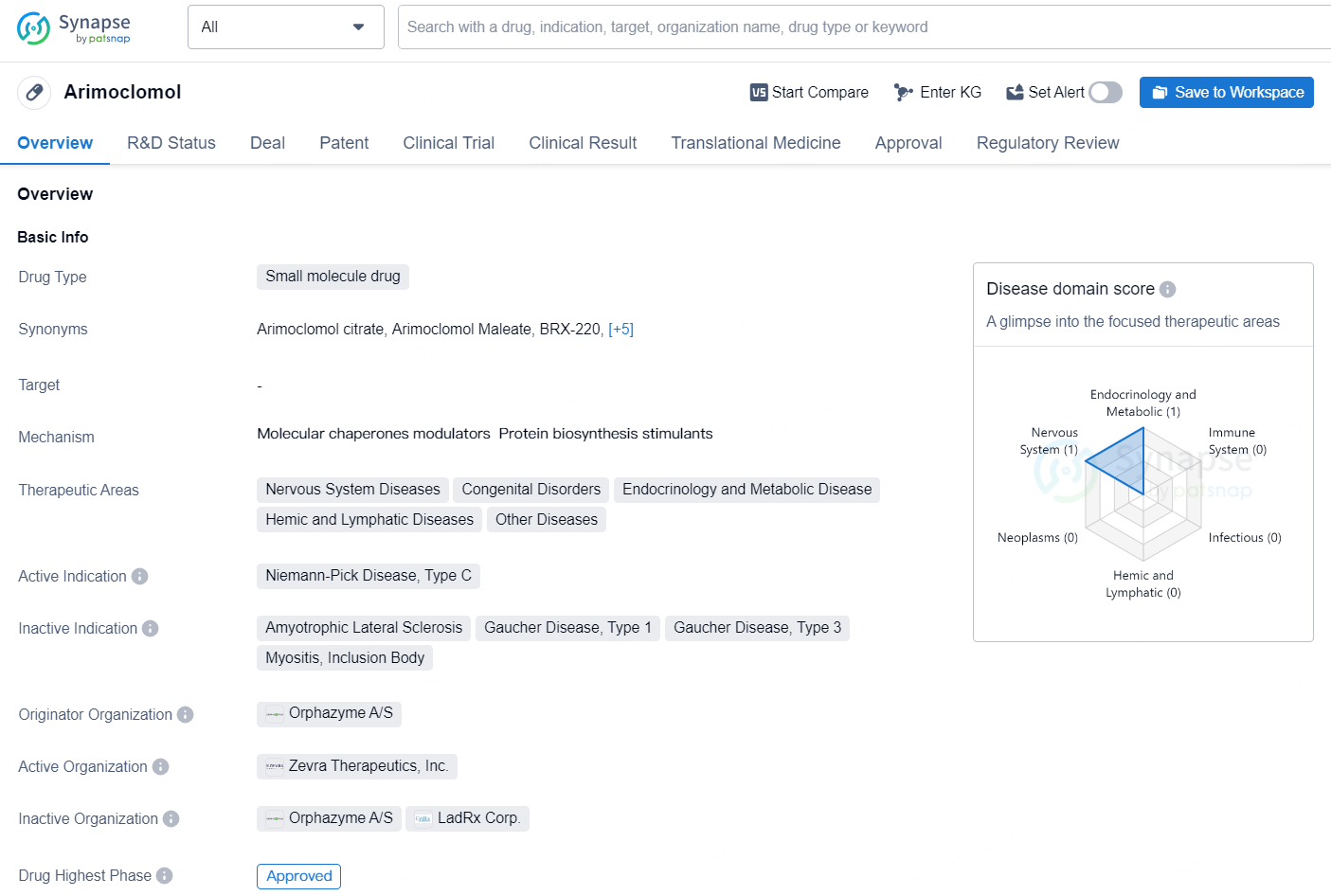
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
NPC is an uncommon genetic disorder leading to progressively worsening neurological and organ function symptoms. This condition arises due to mutations in the NPC1 or NPC2 genes, which disrupt cholesterol and lipid transport within cells. As a consequence, cellular operations are impaired, resulting in organ damage. Typically, individuals with this disorder have a life expectancy of approximately 13 years.
“NPC severely impacts both patients and their families. Despite significant research efforts, no treatments have been approved to address the substantial needs of NPC patients,” stated Janet Maynard, M.D., M.H.S., director of the Office of Rare Diseases, Pediatrics, Urologic, and Reproductive Medicine (ORPURM) at the FDA’s Center for Drug Evaluation and Research. “The first approval of a safe and effective treatment for NPC will be a significant advancement in addressing the critical medical needs of these patients.”
Miplyffa's application was the primary focus at the Genetic Metabolic Diseases Advisory Committee's (GeMDAC) first meeting in August, following the committee's establishment in December 2023. GeMDAC advises on products for diagnosing, preventing, or treating genetic metabolic disorders.
Miplyffa's safety and effectiveness were assessed in a 12-month, randomized, double-blind, placebo-controlled trial involving patients aged 2 to 19 with a confirmed molecular diagnosis of NPC. Fifty patients were assigned in a 2:1 ratio to receive weight-adjusted Miplyffa (31 to 124 mg) or a placebo orally three times daily. Of these, 39 patients (78%) were treated with miglustat as part of the trial's background therapy.
The efficacy of Miplyffa was evaluated using the rescored 4-domain NPC Clinical Severity Scale (R4DNPCCSS) among patients on miglustat during the trial. The R4DNPCCSS assesses disease progression based on ambulation, speech, swallowing, and fine motor skills, with higher scores indicating greater disease severity. Miplyffa showed a slower progression of the disease compared to the placebo, as measured by the R4DNPCCSS score.
The prescribing information for Miplyffa includes warnings about hypersensitivity reactions such as hives and angioedema (swelling beneath the skin). Patients experiencing these reactions should discontinue use. Pregnant women or those planning to become pregnant should not use Miplyffa.
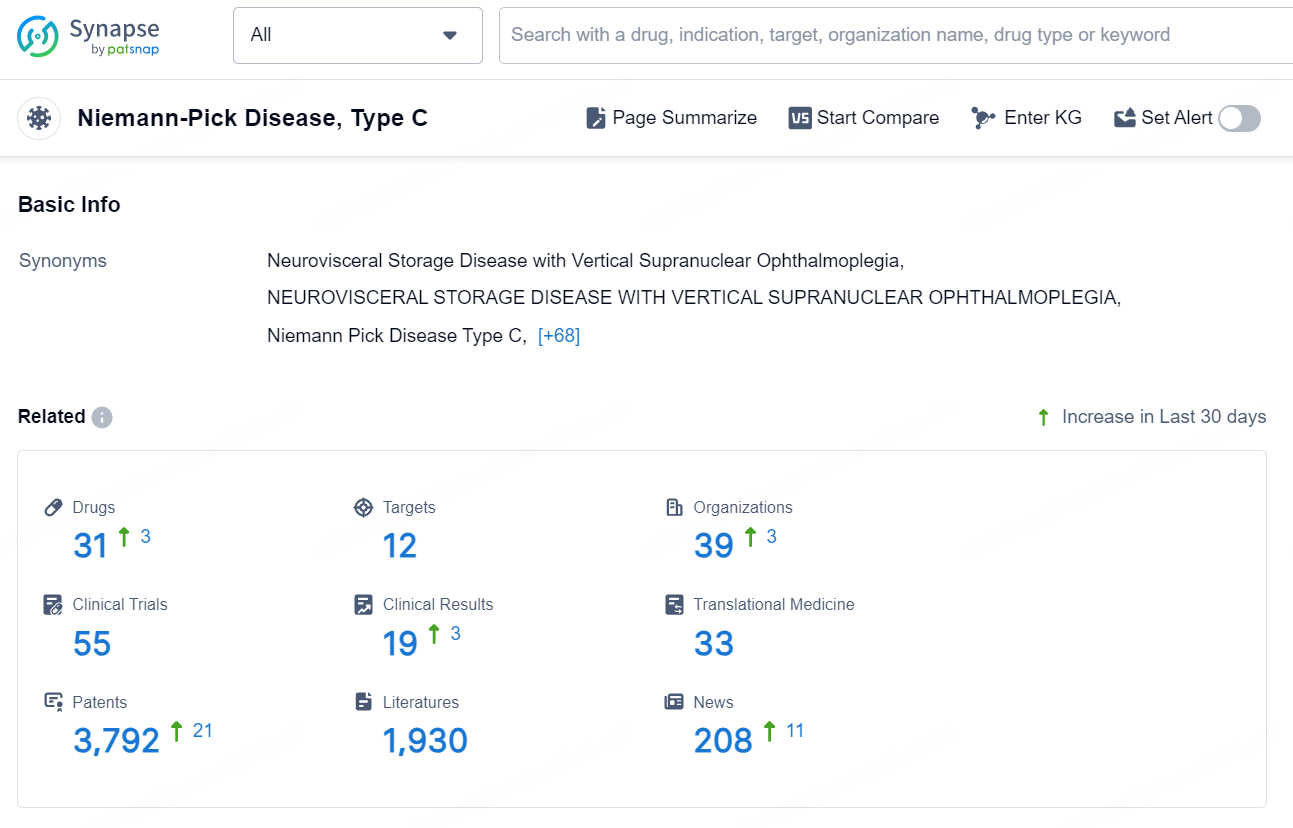
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 9, 2024, there are 31 investigational drugs for the Niemann-Pick Disease, including 12 targets, 39 R&D institutions involved, with related clinical trials reaching 55, and as many as 3792 patents.
Arimoclomol is a small molecule drug developed by Orphazyme A/S and is primarily targeted for the treatment of Niemann-Pick Disease, Type C, a rare genetic disorder that affects the body's ability to metabolize lipids within cells. The drug is classified as a treatment for Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Disease, Hemic and Lymphatic Diseases, and Other Diseases.