Tolebrutinib Delays Disability Progression by 31% in Phase 3 MS Study
The HERCULES phase 3 study yielded positive outcomes in individuals with non-relapsing secondary progressive multiple sclerosis (nrSPMS), showing that tolebrutinib reduced the time to the start of 6-month confirmed disability progression (CDP) by 31% compared to placebo (HR 0.69; 95% CI 0.55-0.88; p=0.0026). Additional analysis of secondary endpoints indicated that the proportion of participants who achieved confirmed disability improvement nearly doubled, from 5% with placebo to 10% with tolebrutinib (HR 1.88; 95% CI 1.10 to 3.21; nominal p=0.021). These findings were revealed in a late-breaking presentation at the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) 2024 conference held in Copenhagen, Denmark.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Houman Ashrafian, MD, PhD
Head of Research & Development, Sanofi
"Currently, there are no treatments available for the wide range of patients suffering from secondary progressive multiple sclerosis. Tolebrutinib has shown promise in slowing down disability progression by targeting the fundamental causes of the disease. We are eagerly awaiting discussions with healthcare authorities about these findings and are keen to observe the results of tolebrutinib in primary progressive MS, anticipated next year. We express our profound gratitude to the trial participants, their families, and the healthcare professionals who contributed to these studies."
The findings from the phase 3 GEMINI 1 and 2 studies of tolebrutinib in comparison to the standard-of-care treatment, Aubagio (teriflunomide), among participants with relapsing multiple sclerosis (RMS) were also unveiled today at ECTRIMS as a late-breaking presentation. Although both studies did not achieve their primary goal of significantly improving annualized relapse rates (ARR) compared to Aubagio, in a key secondary endpoint—a combined analysis from GEMINI 1 and 2— tolebrutinib delayed the onset of 6-month confirmed disability worsening (CDW) by 29% (HR 0.71; 95% CI: 0.53-0.95; p=0.023). This result in delaying CDW by 29% for RMS patients aligns with a 31% delay in CDP observed in patients with nrSPMS. Tolebrutinib's substantial impact on disability accumulation, as opposed to its lack of statistically superior effect on relapses compared to Aubagio, suggests that it may be addressing underlying neuroinflammation that contributes to disease progression independently of relapses.
Moreover, in both GEMINI 1 and 2, the ARR in the Aubagio groups was historically low, and no differences were noted between Aubagio and tolebrutinib in the combined analysis. These relapse rates are approximately equivalent to one relapse every 8 years.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
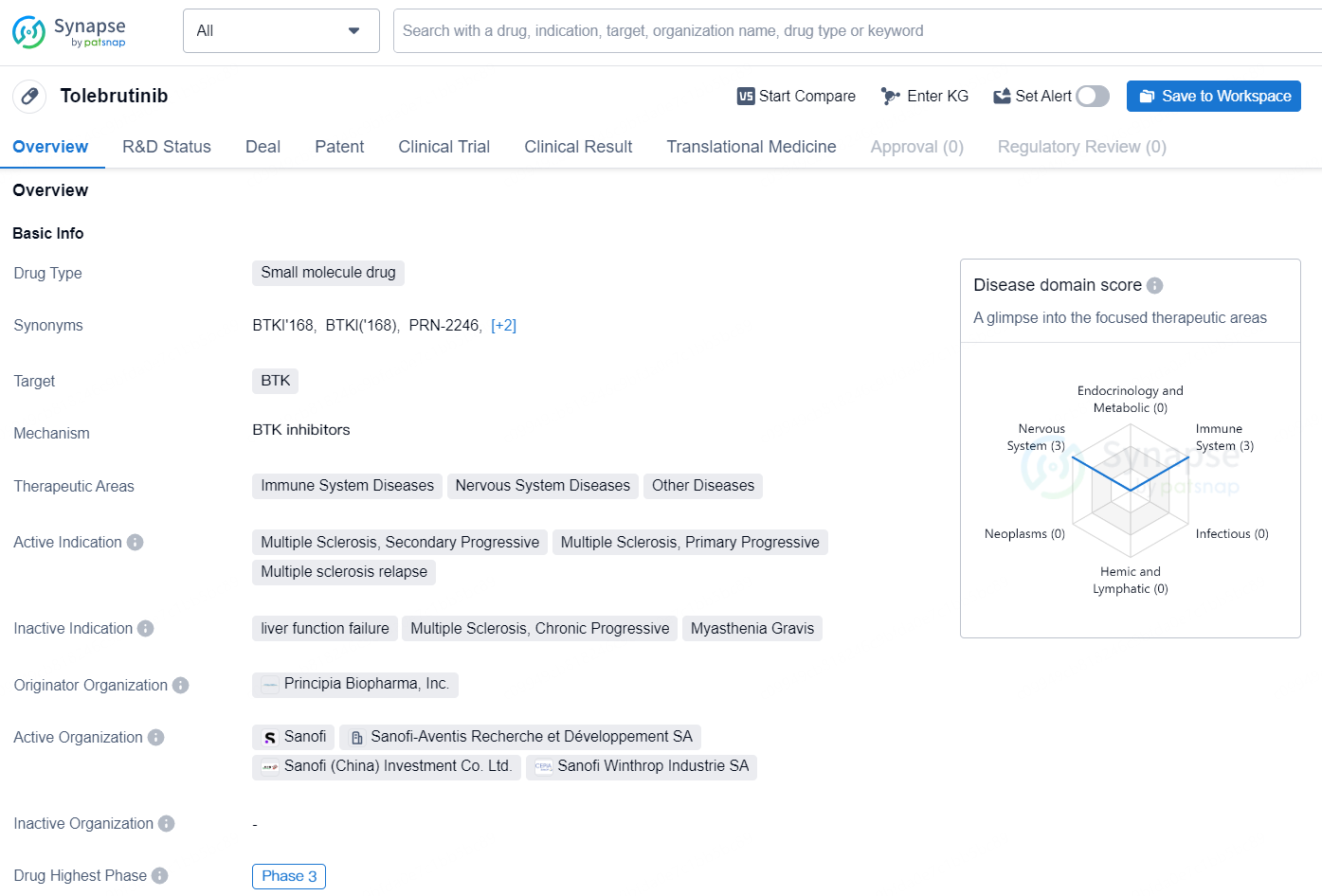
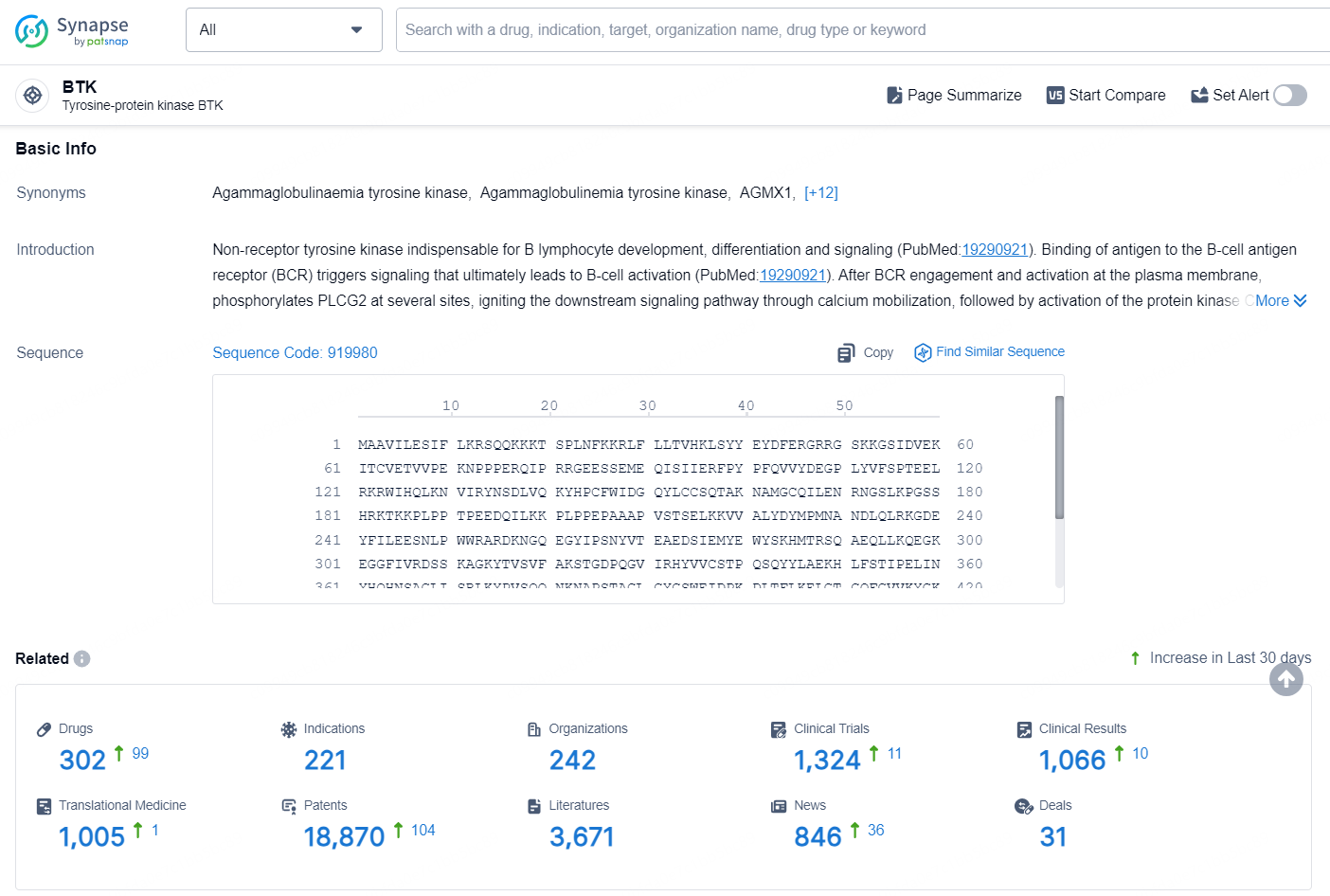
According to the data provided by the Synapse Database, As of September 24, 2024, there are 302 investigational drugs for the BTK target, including 221 indications, 242 R&D institutions involved, with related clinical trials reaching 1324, and as many as 18870 patents.
Tolebrutinib is a small molecule drug that targets the BTK (Bruton's tyrosine kinase) enzyme. It is being developed for the treatment of various therapeutic areas, including immune system diseases, nervous system diseases, and other diseases. The drug is currently in the highest phase of clinical development, which is Phase 3, both globally and in China.