Final Survival Outcomes from TROPION-Breast01 Phase 3 Trial of Datopotamab Deruxtecan
The main findings from the TROPION-Breast01 phase 3 clinical trial evaluating datopotamab deruxtecan (Dato-DXd) against the investigator's choice of chemotherapy, which had earlier met the dual primary endpoint of progression-free survival (PFS), failed to reach statistical significance in the final analysis of overall survival (OS) in patients with inoperable or metastatic hormone receptor (HR) positive, HER2 low or negative (IHC 0, IHC 1+ or IHC 2+/ISH-) breast cancer who had previously undergone endocrine-based therapy and at least one other systemic treatment.
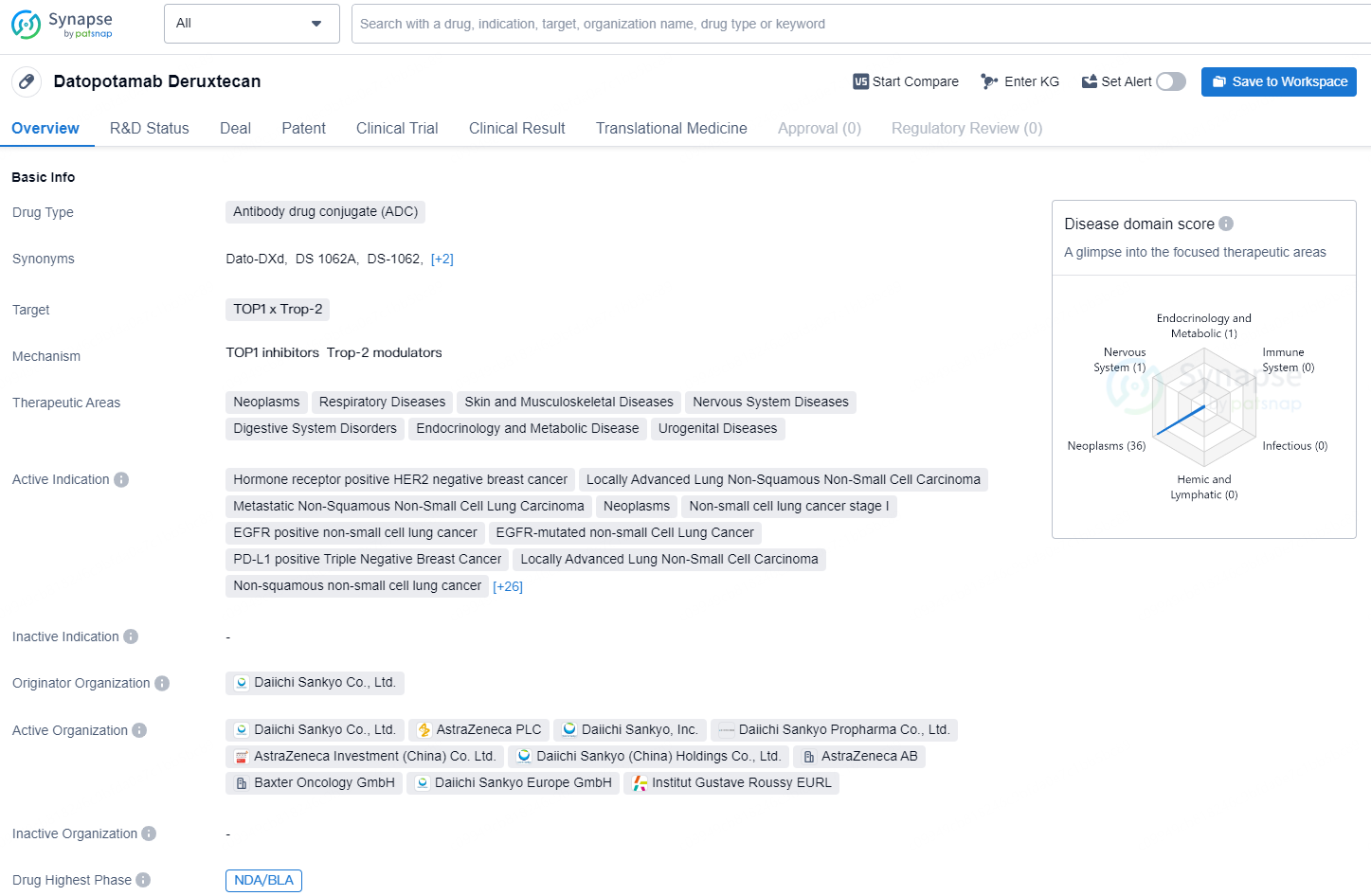
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Datopotamab deruxtecan, a TROP2 targeted DXd antibody drug conjugate (ADC), is a specifically engineered compound discovered by Daiichi Sankyo (TSE: 4568) and is undergoing joint development by Daiichi Sankyo and AstraZeneca (LSE/STO/Nasdaq: AZN).
This review follows the promising progression-free survival (PFS) outcomes revealed at the 2023 European Society for Medical Oncology Congress. The findings indicated that datopotamab deruxtecan showed a statistically significant and clinically important enhancement in PFS, alongside improvements in patient-reported outcomes.1 These PFS data and other vital secondary endpoint results were released this month in the Journal of Clinical Oncology.
The safety profile of datopotamab deruxtecan aligned with previous observations, showing fewer grade 3 or higher treatment-related adverse events in comparison to chemotherapy, and no new safety issues were detected. Interstitial lung disease (ILD) rates remained low across all grades, with no new occurrences of grade 3 or higher ILD.
The trial saw approvals of other ADCs, including ENHERTU® (trastuzumab deruxtecan). Subsequent treatments after disease progression or treatment cessation could have influenced survival outcomes.
"Datopotamab deruxtecan has previously demonstrated a statistically significant progression-free survival advantage in the TROPION-Breast01 trial, corroborated by several significant secondary measures such as patient-reported outcomes," said Ken Takeshita, MD, Global Head of R&D at Daiichi Sankyo. "We take pride in establishing a new standard of care for metastatic breast cancer patients with ENHERTU and remain dedicated to making datopotamab deruxtecan another viable option for those in need."
"The therapeutic landscape for metastatic HR positive breast cancer has greatly advanced in recent years, benefiting patients," noted Susan Galbraith, MBBChir, PhD, Executive Vice President of Oncology R&D at AstraZeneca. "Based on the TROPION-Breast01 findings, datopotamab deruxtecan has shown clinical value in this context. We will maintain discussions with regulatory authorities and integrate insights from these results into our clinical development program for datopotamab deruxtecan in breast cancer."
The data will be presented at an upcoming medical conference and provided to regulatory authorities currently assessing applications for this use.
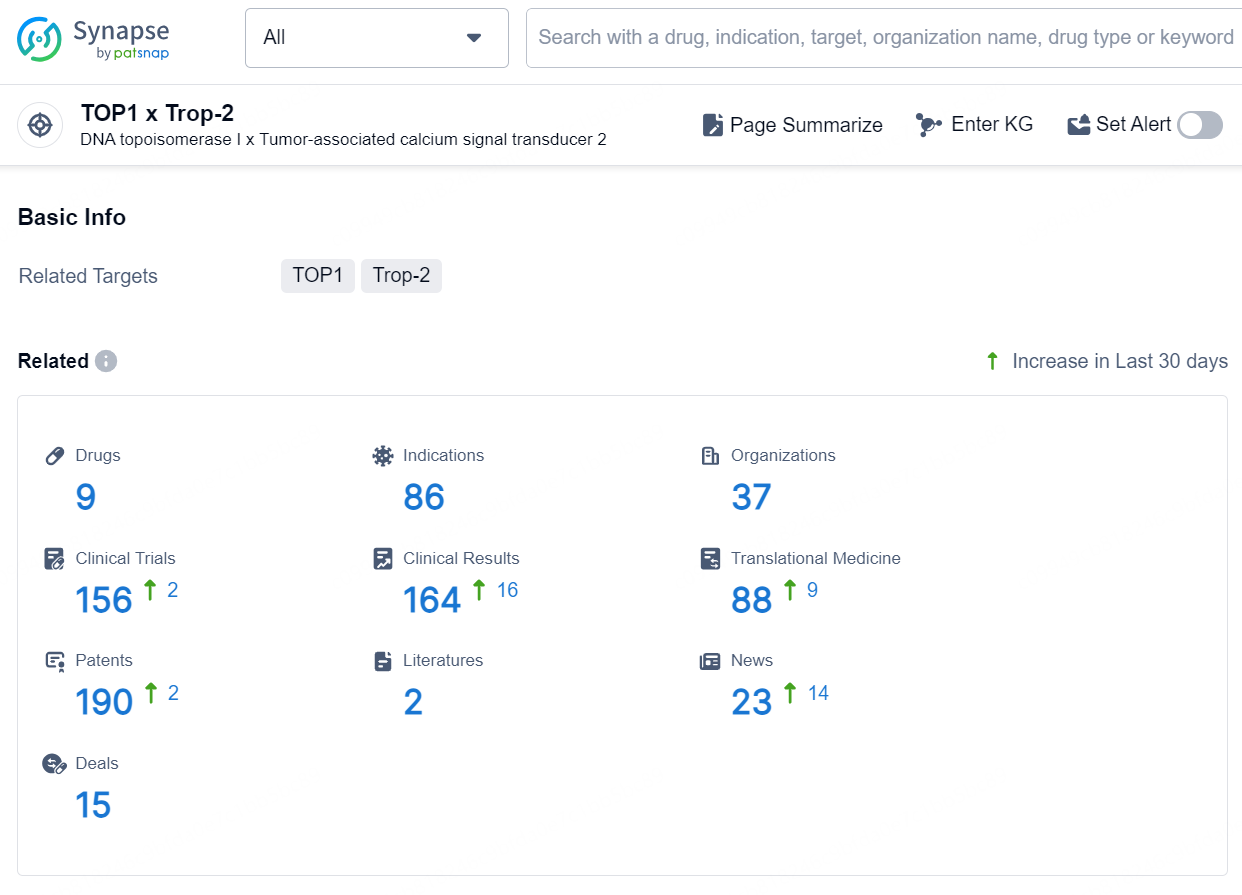
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 24, 2024, there are 9 investigational drusg for the TOP1 x Trop-2 targets, including 86 indications, 37 R&D institutions involved, with related clinical trials reaching 156, and as many as 190 patents.
Datopotamab Deruxtecan is an antibody drug conjugate (ADC) designed to target TOP1 x Trop-2. It is indicated for the treatment of various therapeutic areas, including neoplasms, respiratory diseases, skin and musculoskeletal diseases, nervous system diseases, digestive system disorders, endocrinology and metabolic disease, and urogenital diseases.