UCB Announces FDA Approval of BIMZELX® (Bimekizumab-bkzx) for Multiple Inflammatory Conditions
UCB, an international biopharmaceutical firm, has announced that the U.S. Food and Drug Administration (FDA) has given its approval for BIMZELX® (bimekizumab-bkzx) to treat adults with active psoriatic arthritis (PsA), adults with active non-radiographic axial spondyloarthritis (nr-axSpA) showing objective signs of inflammation, and adults with active ankylosing spondylitis (AS). Bimekizumab-bkzx stands as the inaugural treatment to gain approval for these three conditions, designed specifically to selectively inhibit two primary cytokines, interleukin 17A (IL-17A) and interleukin 17F (IL-17F), that drive inflammatory processes. These new approved uses follow the first U.S. approval of BIMZELX® in October 2023, for treating moderate-to-severe plaque psoriasis in adults eligible for systemic therapy or phototherapy.
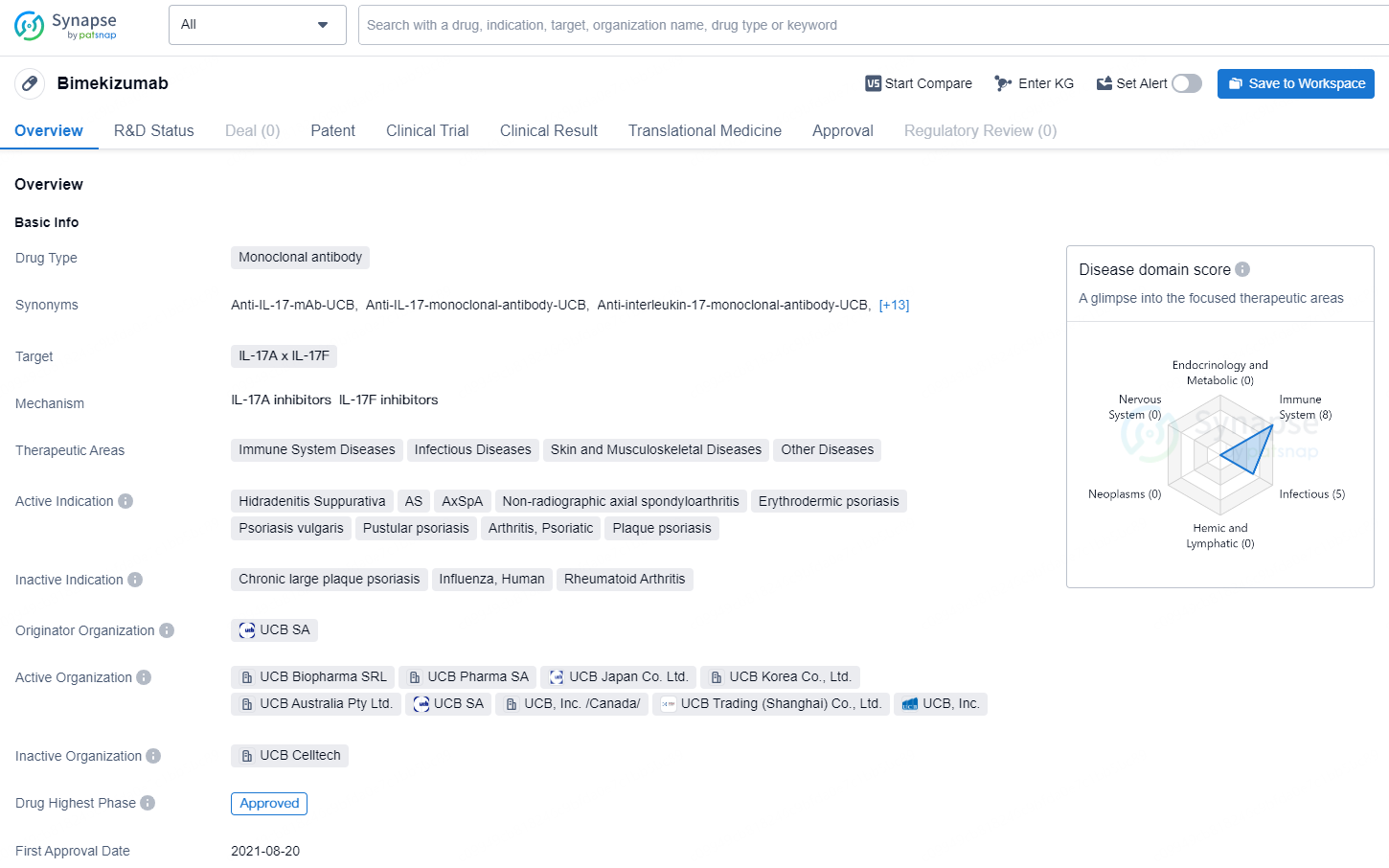
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The U.S. approval of BIMZELX for three new indications – active psoriatic arthritis (PsA), active non-radiographic axial spondyloarthritis (axSpA) with objective signs of inflammation, and active ankylosing spondylitis (AS) – underscores the clinical advantages provided by dual inhibition of IL-17A and IL-17F for patients. This development presents a significant opportunity for more individuals suffering from chronic inflammatory diseases to achieve meaningful clinical outcomes," stated Emmanuel Caeymaex, Executive Vice President, Head of Patient Impact, and Chief Commercial Officer at UCB. "In cases of psoriatic arthritis and the broader axSpA spectrum, clinical trials and real-world data outside the U.S. have demonstrated that BIMZELX can enable patients to reach substantial clinical response thresholds quickly, with benefits that can last up to two years."
The FDA has recommended a dosage of 160 mg bimekizumab via subcutaneous injection every four weeks for adult patients with active PsA, active nr-axSpA with objective signs of inflammation, and active AS. For PsA patients who also have moderate to severe plaque psoriasis, the dosage and administration match those for patients with moderate to severe plaque psoriasis. BIMZELX is currently accessible for eligible patients.
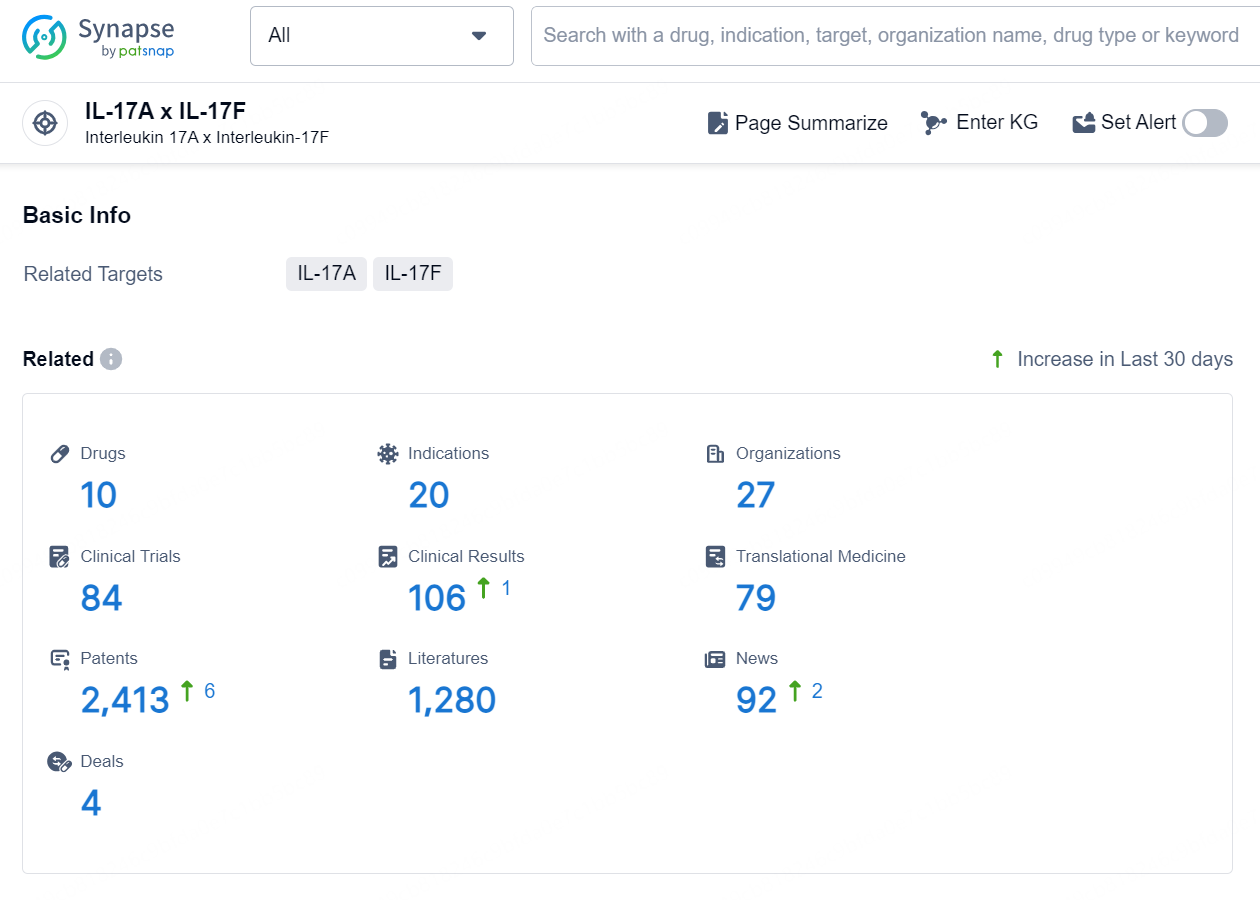
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 24, 2024, there are 10 investigational drugs for the IL-17A and IL-17F targets, including 20 indications, 27 R&D institutions involved, with related clinical trials reaching 84, and as many as 2413 patents.
Bimekizumab is a monoclonal antibody drug that targets IL-17A and IL-17F. It is indicated for the treatment of various immune system diseases, infectious diseases, skin and musculoskeletal diseases, and other conditions. The active indications for Bimekizumab include Hidradenitis Suppurativa, Ankylosing Spondylitis (AS), Axial Spondyloarthritis (AxSpA), Non-radiographic axial spondyloarthritis, Erythrodermic psoriasis, Psoriasis vulgaris, Pustular psoriasis, Psoriatic Arthritis, and Plaque psoriasis.