EU Approves Ipsen's Iqirvo® (elafibranor) as First New Primary Biliary Cholangitis Therapy in a Decade
Ipsen (Euronext: IPN; ADR: IPSEY) announced that the European Commission has granted conditional approval for Iqirvo® (elafibranor) 80mg tablets to treat primary biliary cholangitis (PBC). This treatment can be used in conjunction with ursodeoxycholic acid (UDCA) in adults who do not adequately respond to UDCA or as a stand-alone therapy for those who cannot tolerate UDCA. Iqirvo is an oral, first-in-class peroxisome proliferator-activated receptor (PPAR) agonist, affecting PPARα and PPARδ proteins, which are considered essential in regulating bile acid, inflammation, and fibrosis.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We're thrilled that Iqirvo has received approval in the E.U. as a promising new treatment option for individuals with PBC, a rare liver disease mostly affecting women and lacking significant advances for nearly ten years. This is especially important since up to half of the patients either cannot tolerate current treatments or do not respond to them," stated Sandra Silvestri, Chief Medical Officer at Ipsen. "For those patients at risk of disease progression who continue to suffer from severe symptoms, we're pleased to offer an effective new treatment choice."
The approval of Iqirvo is founded on data from the phase III ELATIVE1 trial, which showed a statistically significant treatment advantage with a 47% placebo-adjusted difference (P<0.001) between patients receiving Iqirvo 80mg (51%) and those on placebo (4%) achieving a biochemical response. Though a more significant reduction in PBC Worst Itch-NRS score from baseline was noted in patients on Iqirvo compared to placebo, it was not statistically significant. Treatment with Iqirvo was linked with improved pruritus (itch) as demonstrated by greater reductions in PBC-40 itch and 5-D itch total scores compared to placebo. The incidence of adverse events, treatment-related adverse events, severe or serious adverse events, and adverse events leading to discontinuation was similar between the Iqirvo group and the placebo group.
"This advancement in PBC treatment is positive news for the effective and well-tolerated management of the condition," remarked Dr. Marco Carbone, Professor of Gastroenterology at the University of Milano-Bicocca and Consultant Hepatologist at the Niguarda Liver Transplant Centre in Milan. "PBC is a progressive liver disease that can result in liver failure or the necessity for liver transplantation in some individuals. Therefore, this new medication, which has shown potential to manage disease progression and reduce itching, a symptom that significantly impacts patients' quality of life, is very encouraging for physicians and their patients."
"PBC is a highly personal disease, impacting each individual differently. While some patients experience extreme fatigue or severe itching, others might display no symptoms but have poor biomarker levels, indicating that their disease is not controlled. This highlights the necessity of a personalized approach to disease management and treatment for each patient," said Patient Advocate Mrs. Sindee Weinbaum from the European Liver Patients’ Association. "Patients must collaborate with their healthcare providers to discuss individualized needs for managing their PBC. Thus, having a new treatment option for many struggling with uncontrolled PBC is excellent news."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
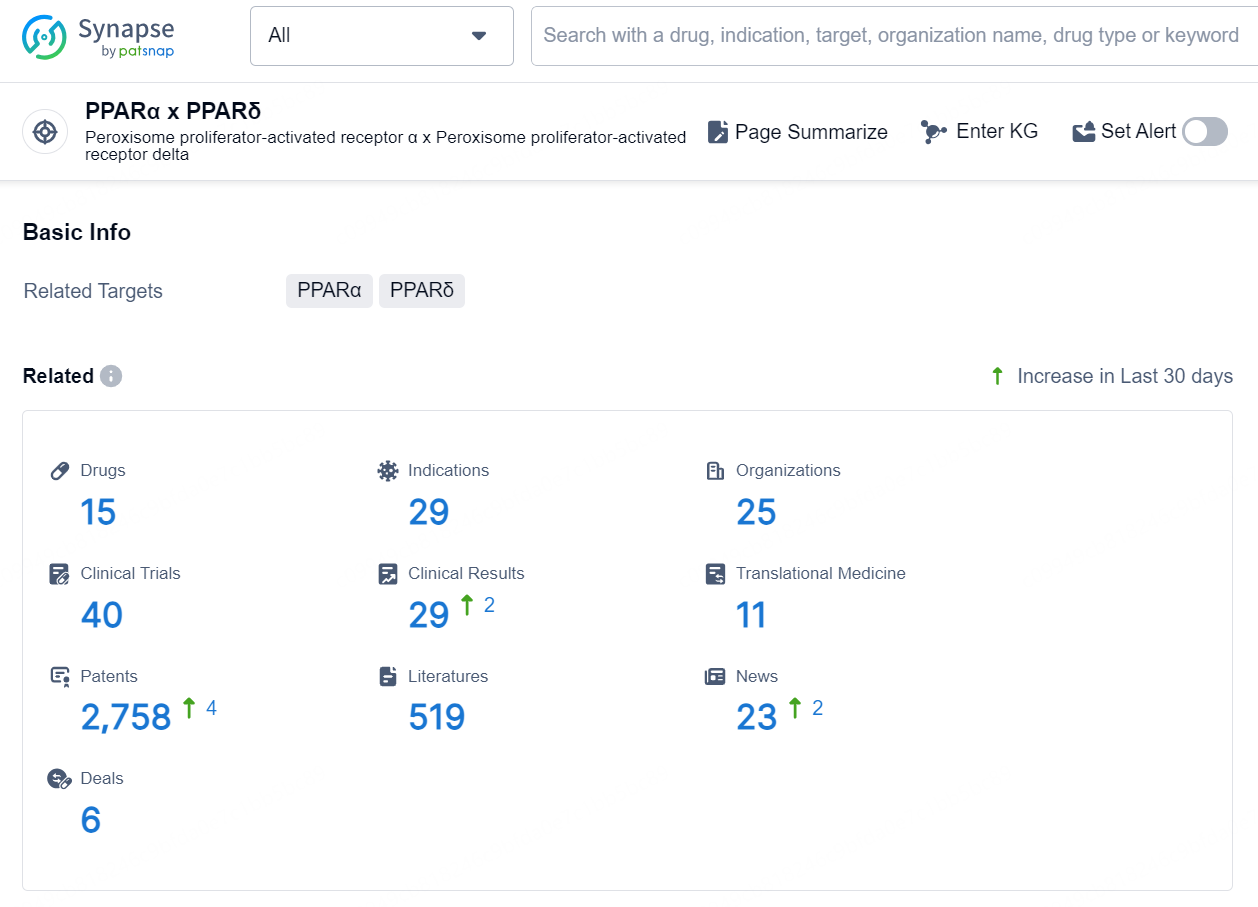
According to the data provided by the Synapse Database, As of September 24, 2024, there are 15 investigational drug for the PPARα and PPARδ targets, including 29 indications, 25 R&D institutions involved, with related clinical trials reaching 40, and as many as 2758 patents.
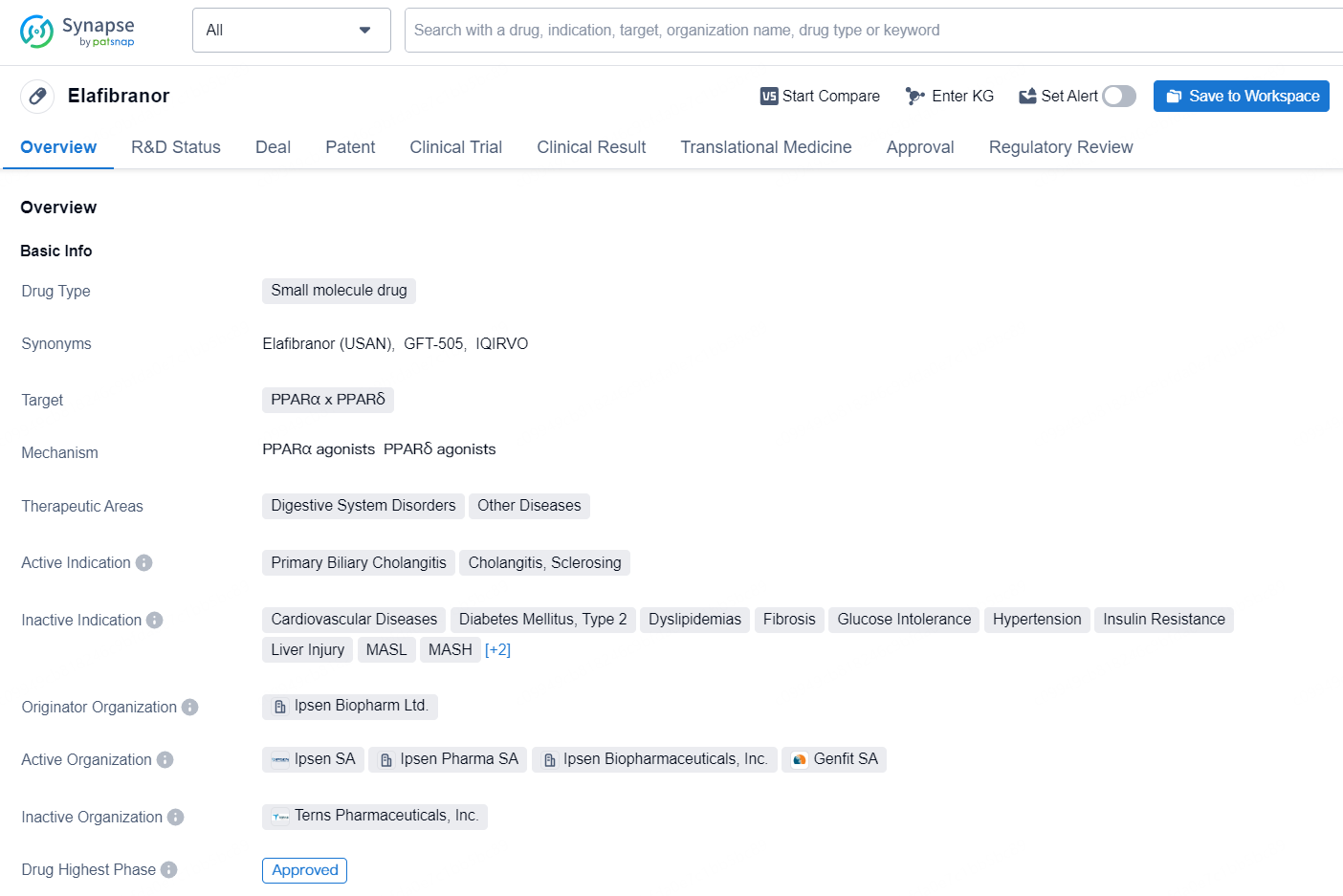
Elafibranor is a small molecule drug that targets PPARα and PPARδ. It is indicated for the treatment of digestive system disorders and other diseases, particularly primary biliary cholangitis, cholangitis, and sclerosing. The drug was developed by Ipsen Biopharm Ltd., and it has reached the highest phase of approval globally, with the first approval date set for June 2024 in the United States.