Finding the Complete Antibody Sequence Using a Known CDR Sequence: An Exploration with Patsnap Bio Sequence Database
One can elucidate the entire antibody sequence by utilizing a known Complementarity-determining region (CDR) sequence in combination with the accessible Patsnap Bio Sequence Database. This resource is complimentary and requires only a simple registration process.
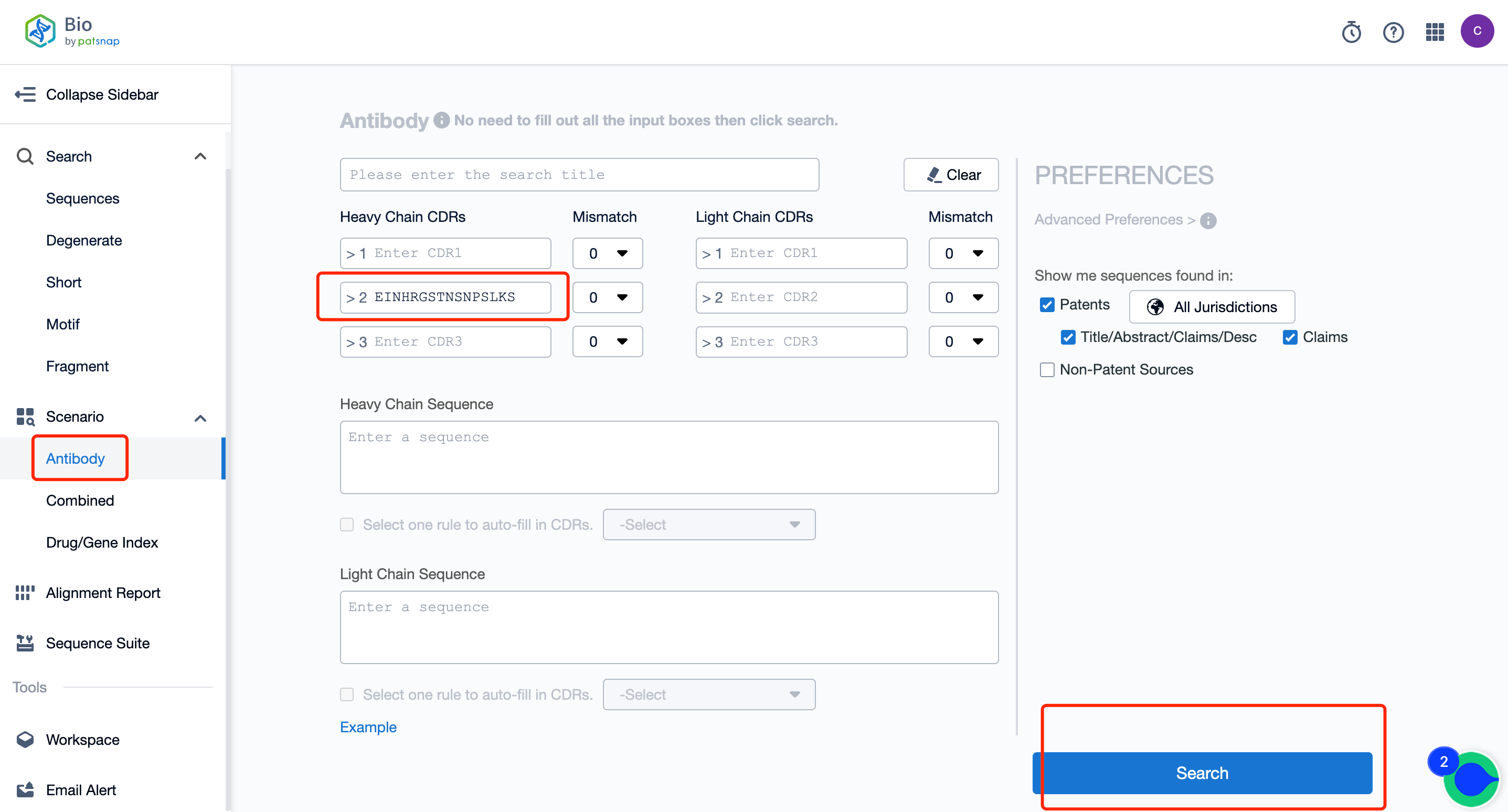
To embark on this journey, access the Bio database account registration here. Upon entering the vibrant interface of the database homepage, look for the antibody search option and input the desired CDR sequence into the search field (Refer to Figure 1 for visual guidance).
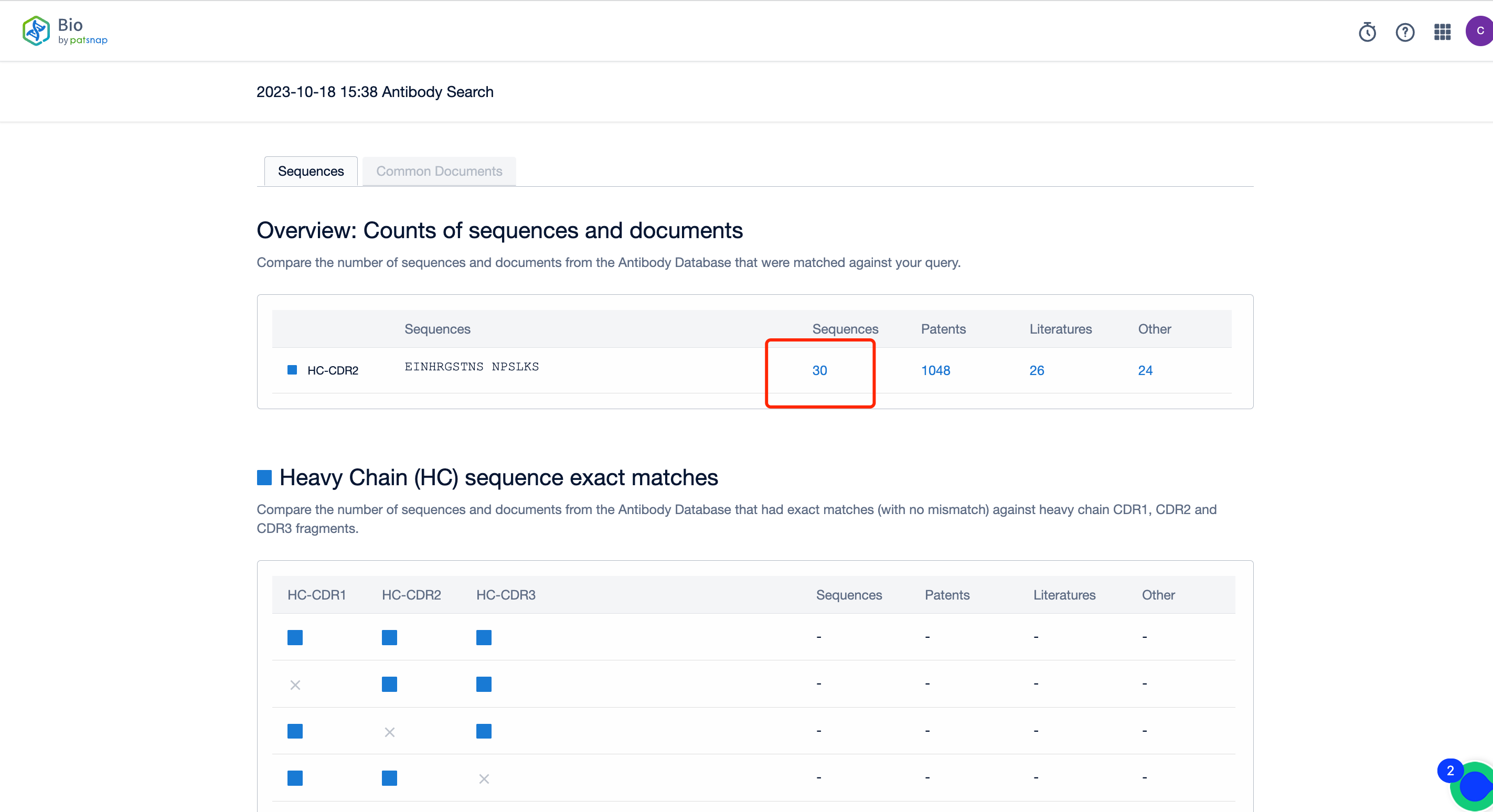
Activating the search button will transport you to the search results page (Outlined in Figure 2). In this arena, you can peruse the number of sequences hit, and review their availability in the public domain. By engaging with the numerical values below the sequence, the gateway to a deep understanding of the hit sequences is unlocked.
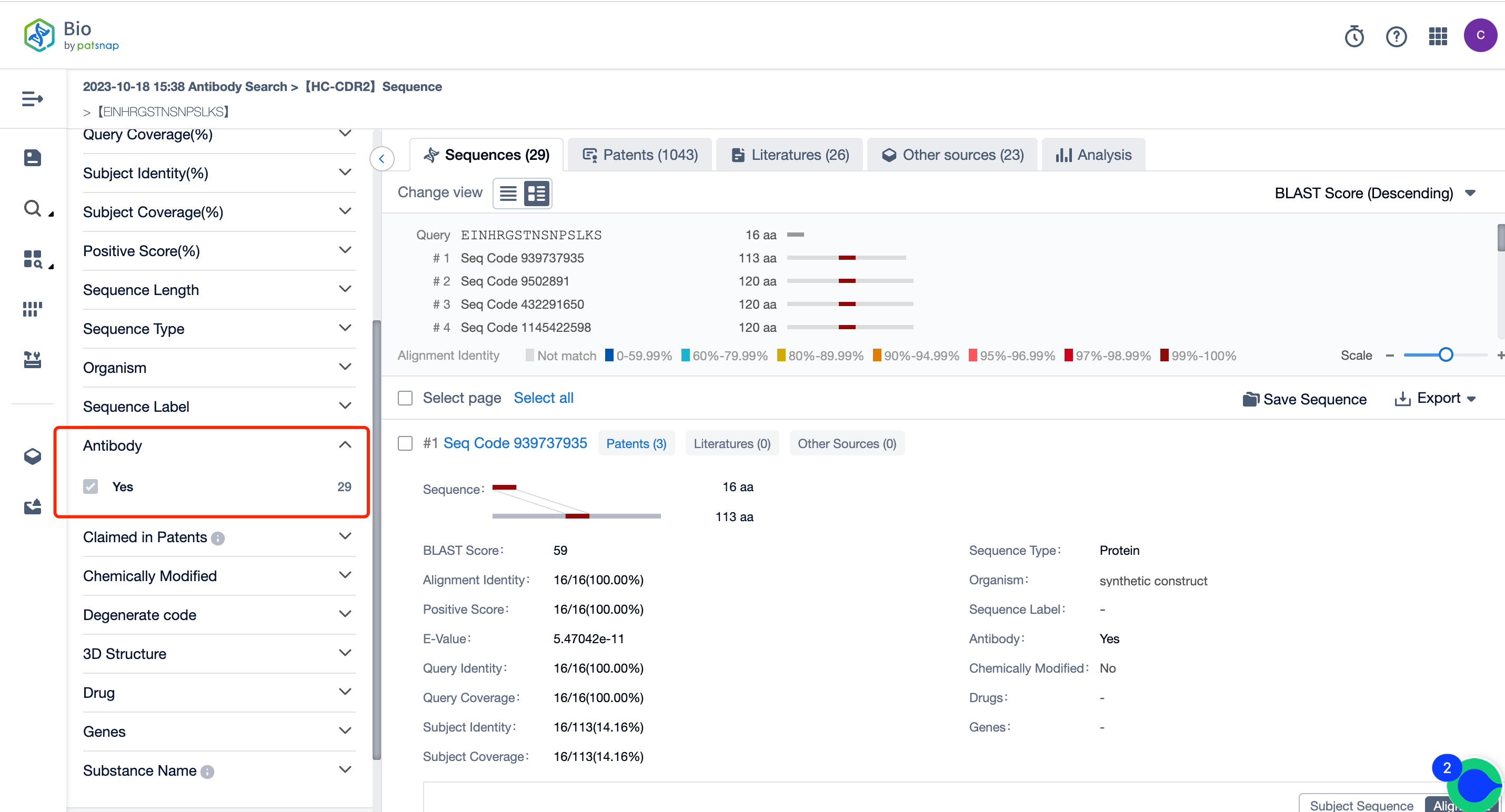
Sifting through the multitude of antibodies will lead to the discovery of the comprehensive hit antibody sequences (Graphically represented in Figure 3). With the foundation of the hit sequences, one can delve further into analysis by examining related patent information. This process could provide valuable insights and enhance the depth of your exploration.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.