Finerenone Enhances Cardiovascular Outcomes in Common Heart Failure
Extensive findings from the Phase III FINEARTS-HF clinical trial reveal that finerenone (Kerendia™/ Firialta™), in comparison to a placebo, offers a statistically significant enhancement in cardiovascular outcomes for patients suffering from heart failure (HF) with a left ventricular ejection fraction (LVEF) of 40% or higher. Finerenone markedly decreased the risk of the combined primary endpoint—comprising cardiovascular death and total (first and recurring) HF incidents, including hospitalizations for HF or emergency HF visits—by 16% (relative risk reduction, rate ratio (RR) 0.84 [95% CI, 0.74-0.95; p=0.0072]) over a median span of 32 months. The FINEARTS-HF results establish finerenone as the first MR antagonist to conclusively show cardiovascular advantages in a Phase III trial for this prevalent type of heart failure.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Heart failure (HF) impacts over 60 million individuals globally, with around half experiencing HF characterized by a left ventricular ejection fraction (LVEF) of ≥40%. This subtype of HF is associated with multiple comorbidities, complicating its management. Current trends suggest this expanding demographic will soon represent the majority of HF-related hospitalizations. Patients with HF and LVEF ≥40% face similar rates of hospitalization and mortality as those with reduced ejection fraction (HFrEF), where LVEF is ≤40%. Notably, over half of these patients are likely to die within five years. Despite available therapies, the residual risk for cardiovascular (CV) events and overall mortality remains elevated in this group.
"Bayer has a longstanding legacy in cardiology, positioning heart failure as a major area of focus for us. These encouraging results reinforce our dedication to aiding patients afflicted by this severe condition. In the FINEARTS-HF study, finerenone demonstrated a reduction in cardiovascular outcomes within a challenging patient cohort. This signifies the potential of finerenone, pending approval, as a viable treatment for heart failure with mildly reduced or preserved ejection fraction, regardless of prior therapies or disease stages," stated Dr. Christian Rommel, Head of R&D at Bayer’s Pharmaceuticals Division. "The FINEARTS-HF study encompassed a significant proportion of patients who were either currently or recently hospitalized, thereby rendering the findings highly pertinent for enhancing cardiovascular outcomes for patients with limited treatment options."
Finerenone acts as a non-steroidal, selective mineralocorticoid receptor (MR) antagonist. By targeting MR and inhibiting the renin-angiotensin-aldosterone system (RAAS) overactivation, finerenone addresses key aspects of HF with LVEF ≥40%, including fibrotic mechanisms.
The FINEARTS-HF study maintained finerenone's established safety profile, and the drug was well-tolerated. The incidence of serious adverse events emerging from treatment was similar between the finerenone and placebo groups. However, hyperkalemia-related adverse events were more prevalent with finerenone compared to placebo (9.7% vs. 4.2%). There were no fatal hyperkalemia events in either group, and hospitalizations or discontinuations due to hyperkalemia were uncommon. Elevated potassium and creatinine levels were more frequent in the finerenone group, though instances of potassium levels surpassing 6.0 mmol/L were generally rare.
Bayer intends to seek marketing authorization for finerenone to treat heart failure with a LVEF of ≥40% from health regulatory authorities in due course.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
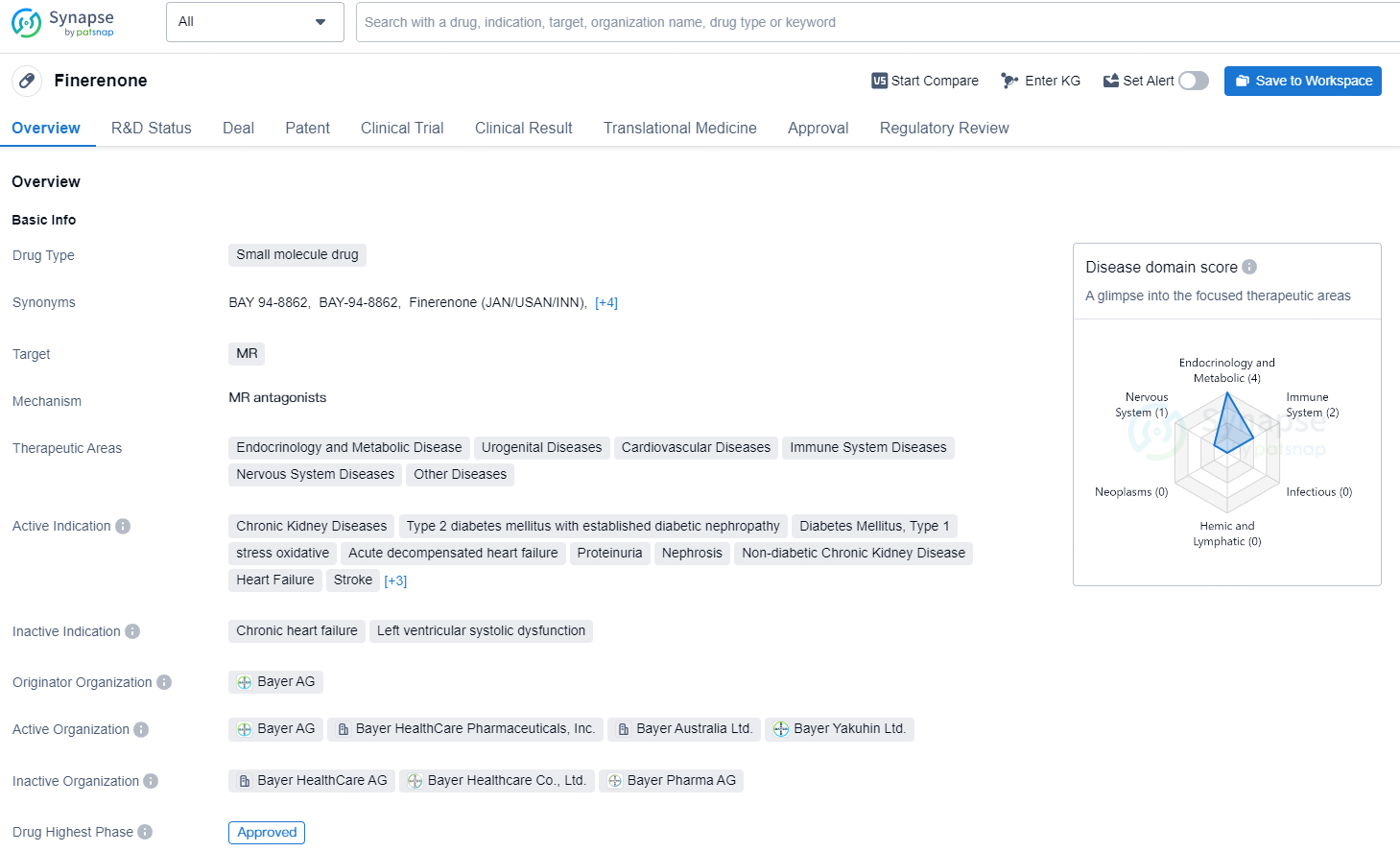
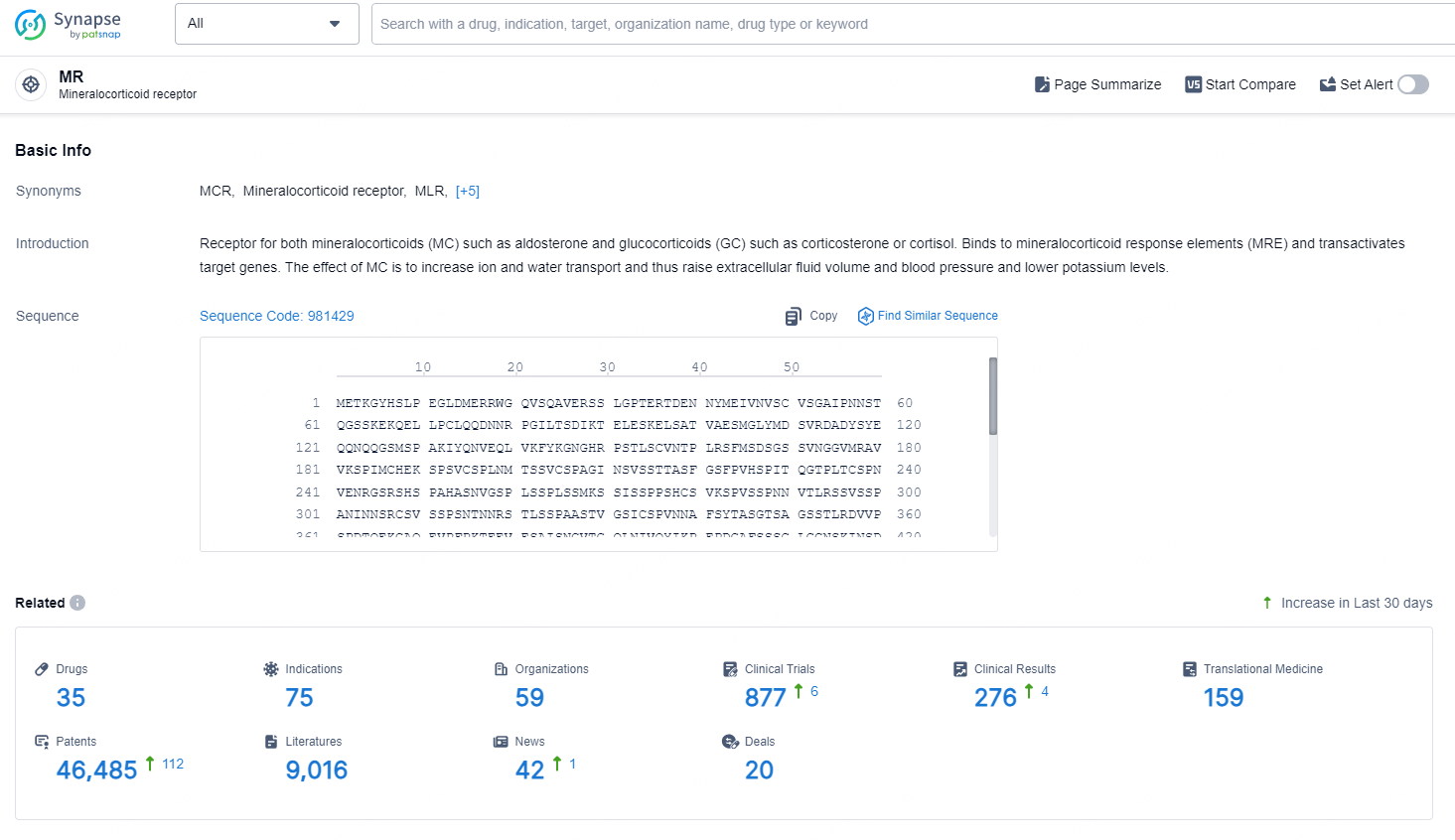
According to the data provided by the Synapse Database, As of September 4, 2024, there are 35 investigational drugs for the MR target, including 75 indications, 59 R&D institutions involved, with related clinical trials reaching 877, and as many as 46485 patents.
Finerenone is a small molecule drug developed by Bayer AG and approved in the United States in July 2021. It targets the mineralocorticoid receptor (MR) and is indicated for various therapeutic areas, including endocrinology and metabolic disease, urogenital diseases, cardiovascular diseases, immune system diseases, nervous system diseases, and other diseases. The drug is approved for the treatment of chronic kidney diseases, type 2 diabetes mellitus with established diabetic nephropathy, type 1 diabetes mellitus with established diabetic nephropathy, oxidative stress, acute decompensated heart failure, proteinuria, nephrosis, non-diabetic chronic kidney disease, heart failure, stroke, transplant complications, type 1 diabetes mellitus with established diabetic nephropathy, and type 2 diabetes mellitus.