Utilize Patsnap Chemical to Unlock the Chemical Structure and Patent Layout of Fulzerasib
Recently, Innovent Biologics announced that Fulzerasib has been approved for marketing by the Center for Drug Evaluation of China's National Medical Products Administration. This drug is indicated for adult patients with advanced non-small cell lung cancer (NSCLC) harboring a KRAS G12C mutation who have received at least one prior systemic therapy, offering new hope for patients with advanced NSCLC.
The process of quickly obtaining market approval for drugs, along with strategies on patent layouts, can help users comprehensively understand drug information and also provide data needs related to new drug research and development, market analysis, and investment decisions for different user roles such as research and development decision-makers, business development experts, and pharmaceutical analysts.
The Synapse database integrates entities and their relationships, including diseases, drugs, targets, and institutions, and constructs a one-stop big data service platform for the pharmaceutical industry using publicly available data from patents, papers, clinical trials, and news. This platform provides a very convenient and quick way to access information about drug development processes like clinical trials and outcomes, and transaction details. Meanwhile, Patsnap Chemical offers in-depth analyses of drug development, patent analysis, and risk assessment, providing comprehensive data support for drug research and development.
Below, we will use the drug Fulzerasib as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Synapse database and Patsnap Chemical.
Step One: Utilize the Synapse database to obtain research and development progress on Fulzerasib
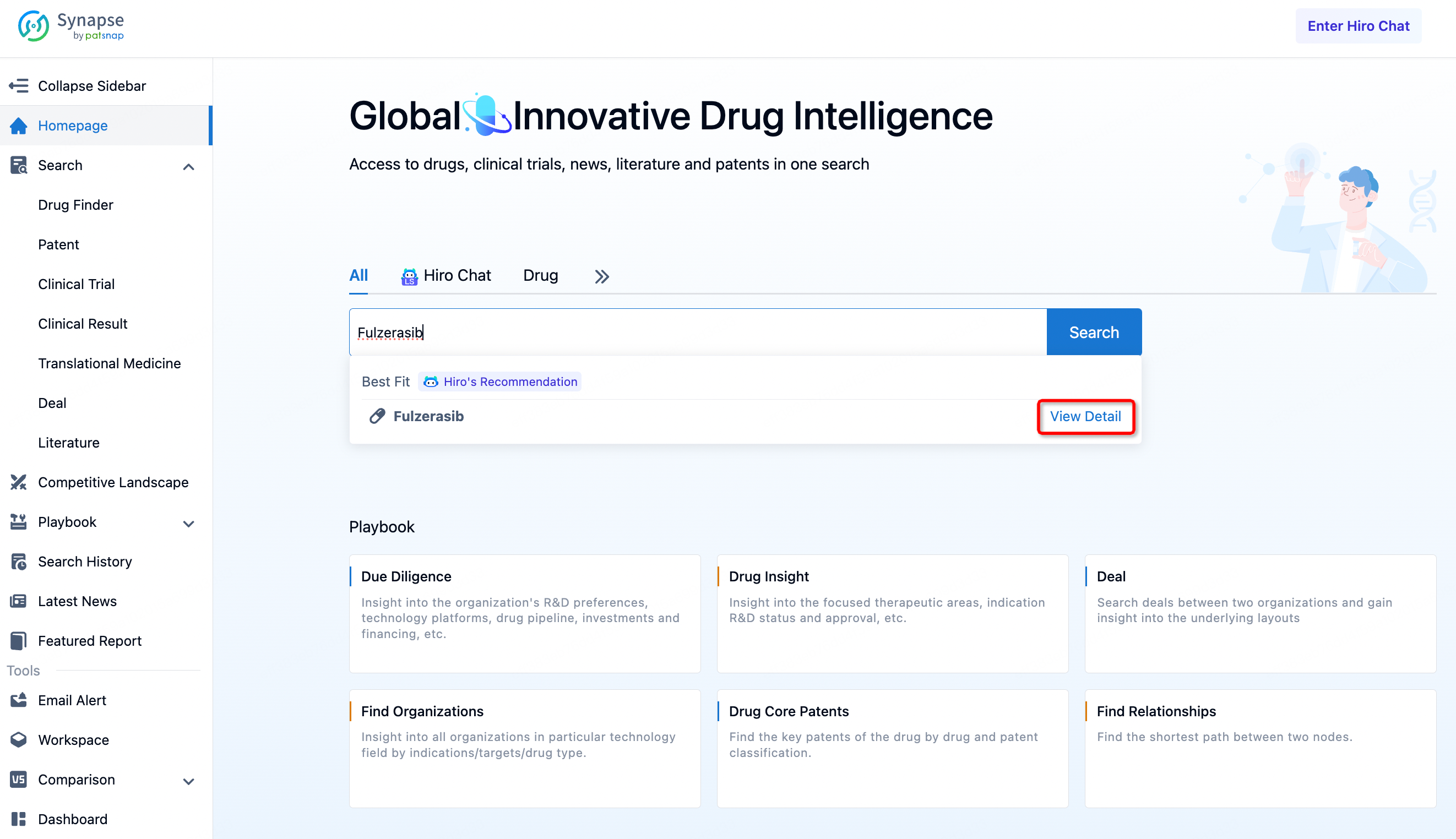
Log in to the Synapse database, and directly enter "Fulzerasib" into the search bar on the homepage. Additionally, searches can also be conducted based on actual needs through drug, patent, clinical trial, and drug transaction information.
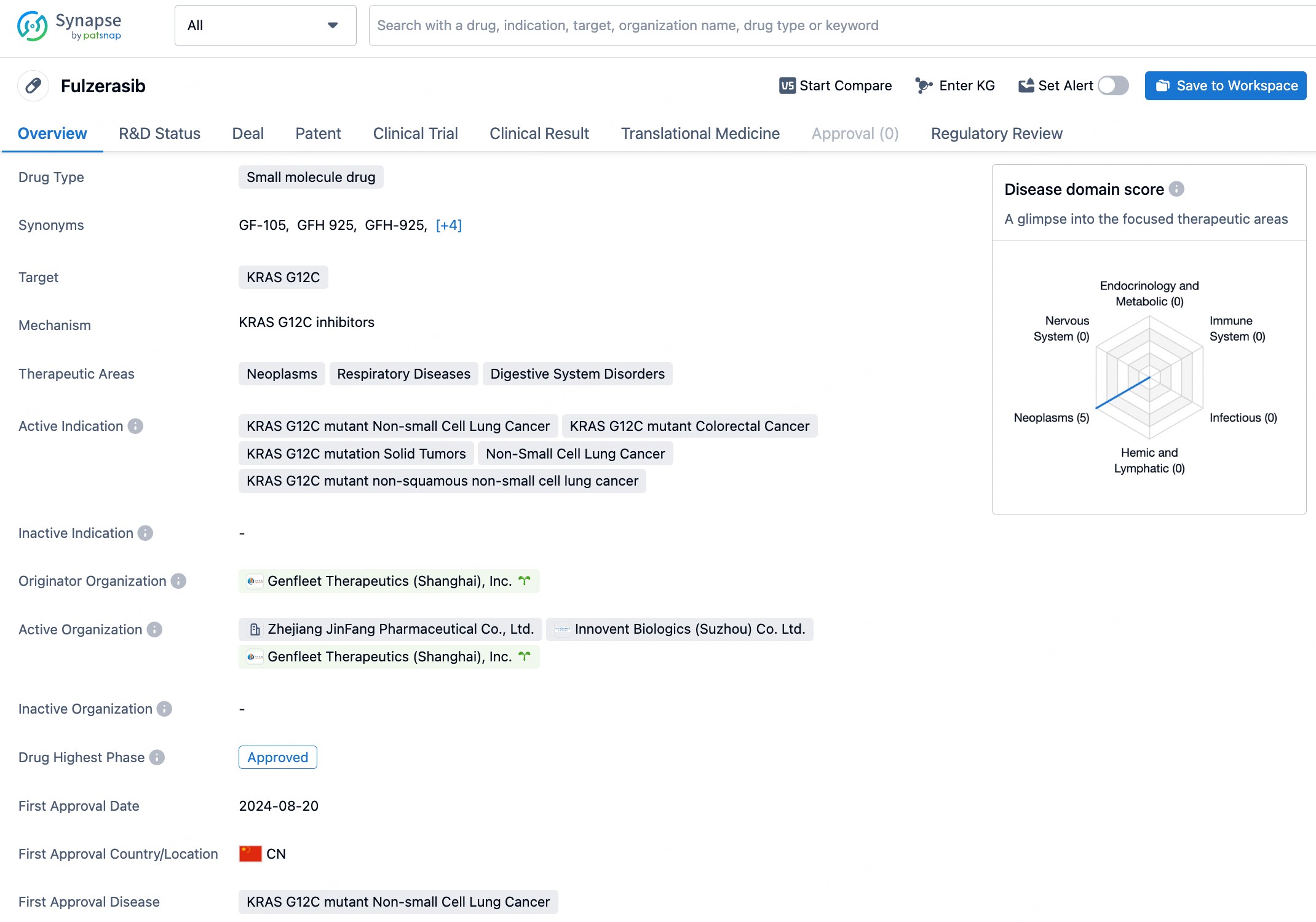
Clicking on "view detail" provides access to various information related to Fulzerasib. From the information displayed, we can see categories including drug details, drug transactions, news, clinical trials, clinical outcomes, and patents. Users can acquire different pieces of information about the drug according to their specific needs. For example, by clicking on patents for more details, we can see the core patent related to Fulzerasib, which bears the application number CN202080034074.0 and was granted on August 5, 2022 (patent publication number CN113853373B).
For chemical drugs, you can directly obtain the chemical structure of the drug on the drug detail page. Click the "find similar" button to directly jump to Patsnap Chemical for similarity search.
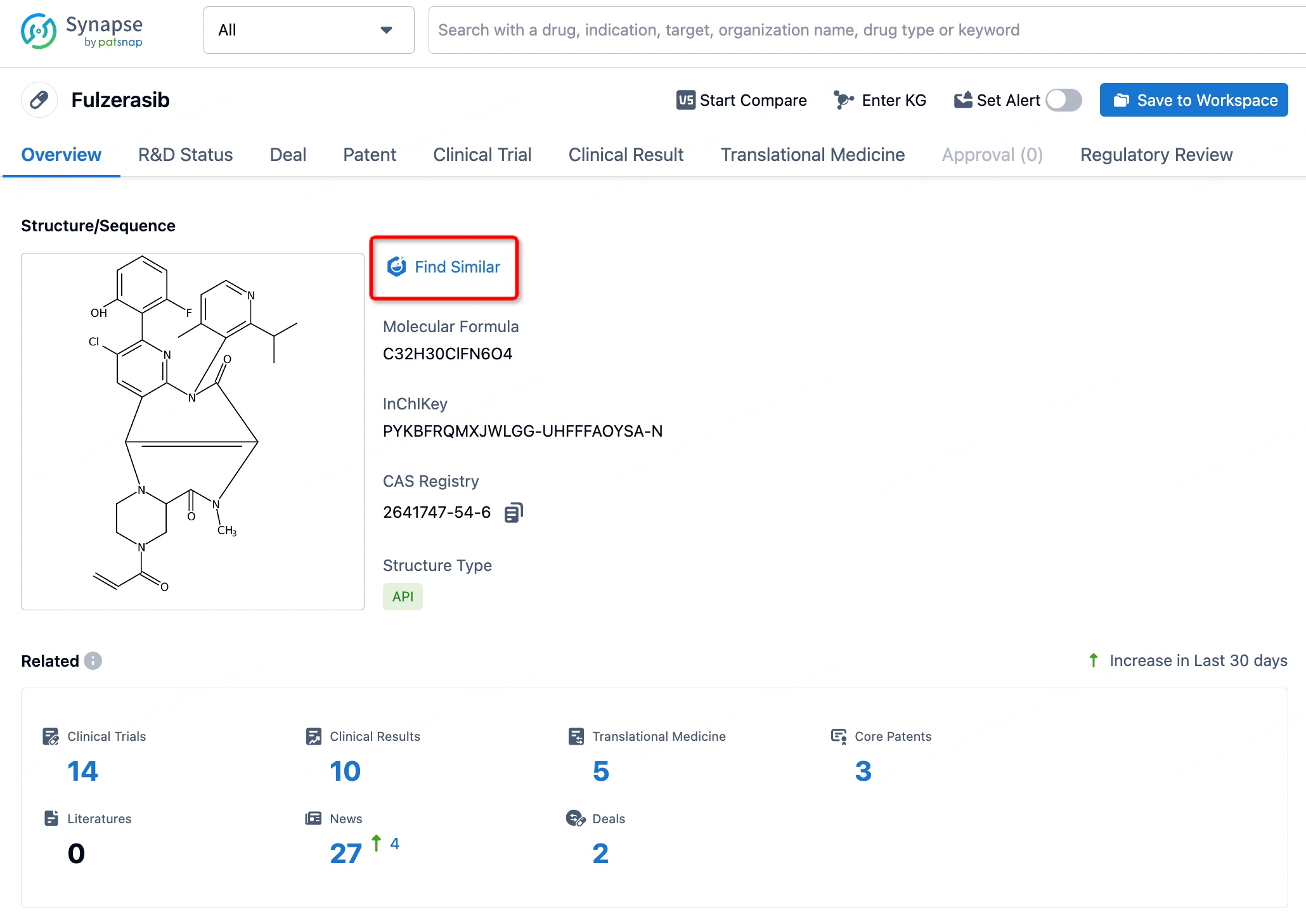
Step Two: Conduct searches for similar patents and literature through Patsnap Chemical
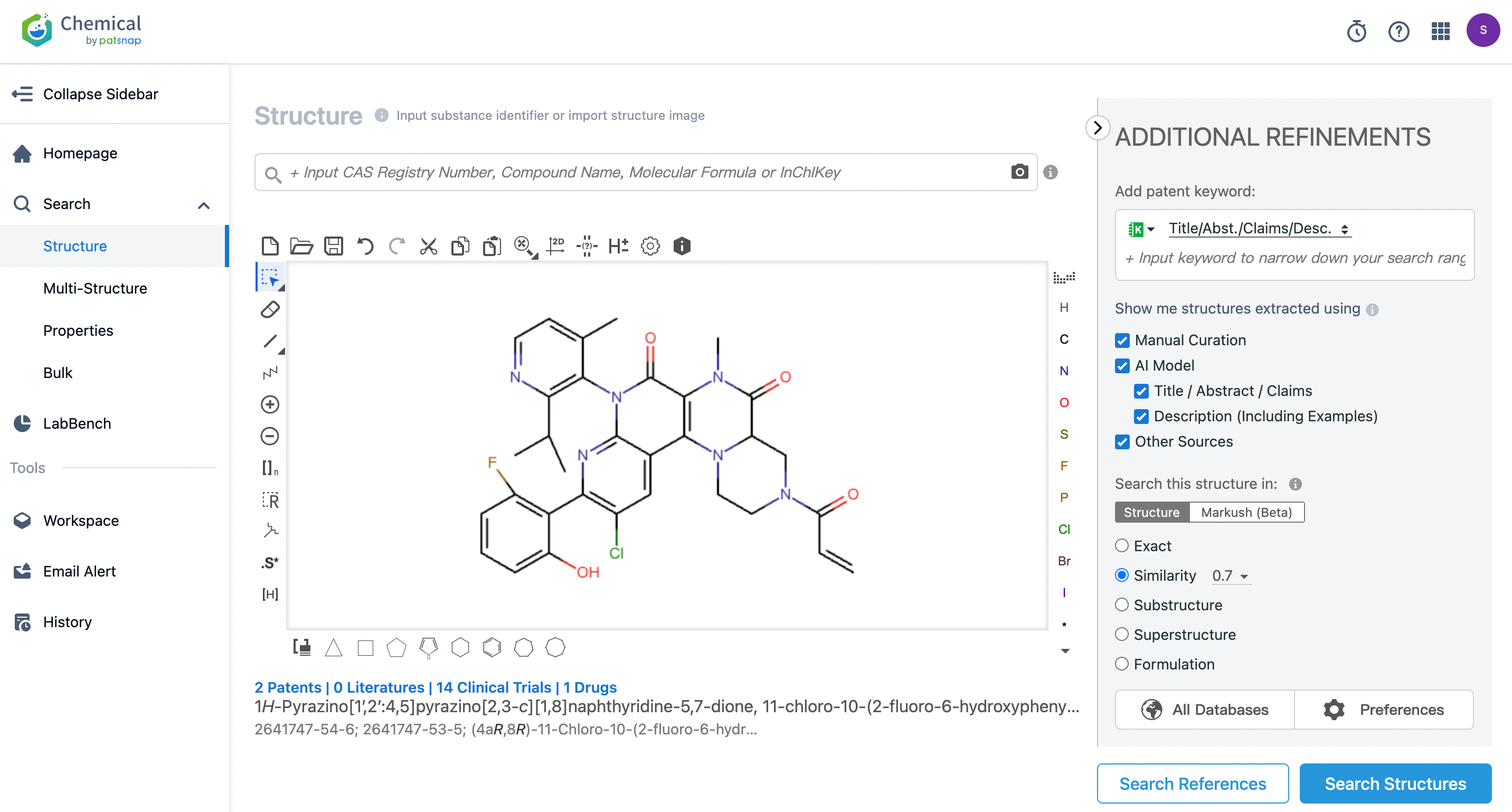
After switching to Patsnap Chemical, searches for similar patents and publications can be conducted by setting search criteria.
Of course, you can also directly search on Patsnap Chemical. After logging in to the Patsnap Chemical homepage, select the structural search and enter the common identity information for Fulzerasib (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Additionally, structures can be edited directly in the structure editor box, and it is also possible to upload structure files drawn in ChemDraw (in MOL format). For core compound searches of drugs, you can select manually verified data sources to ensure the accuracy of the search results. For targeted searches, you can also choose precise search and click to search the compound directly. Here, using a similarity search (setting the Tanimoto coefficient to 0.7), we search for patent applications related to structures similar to Fulzerasib.
By reviewing the search results, we can directly obtain the distribution of patents and literature associated with compounds structurally similar to Fulzerasib. Clicking on the compound name allows quick access to basic information about the compound, clinical trial data, source details, functional information, and more.
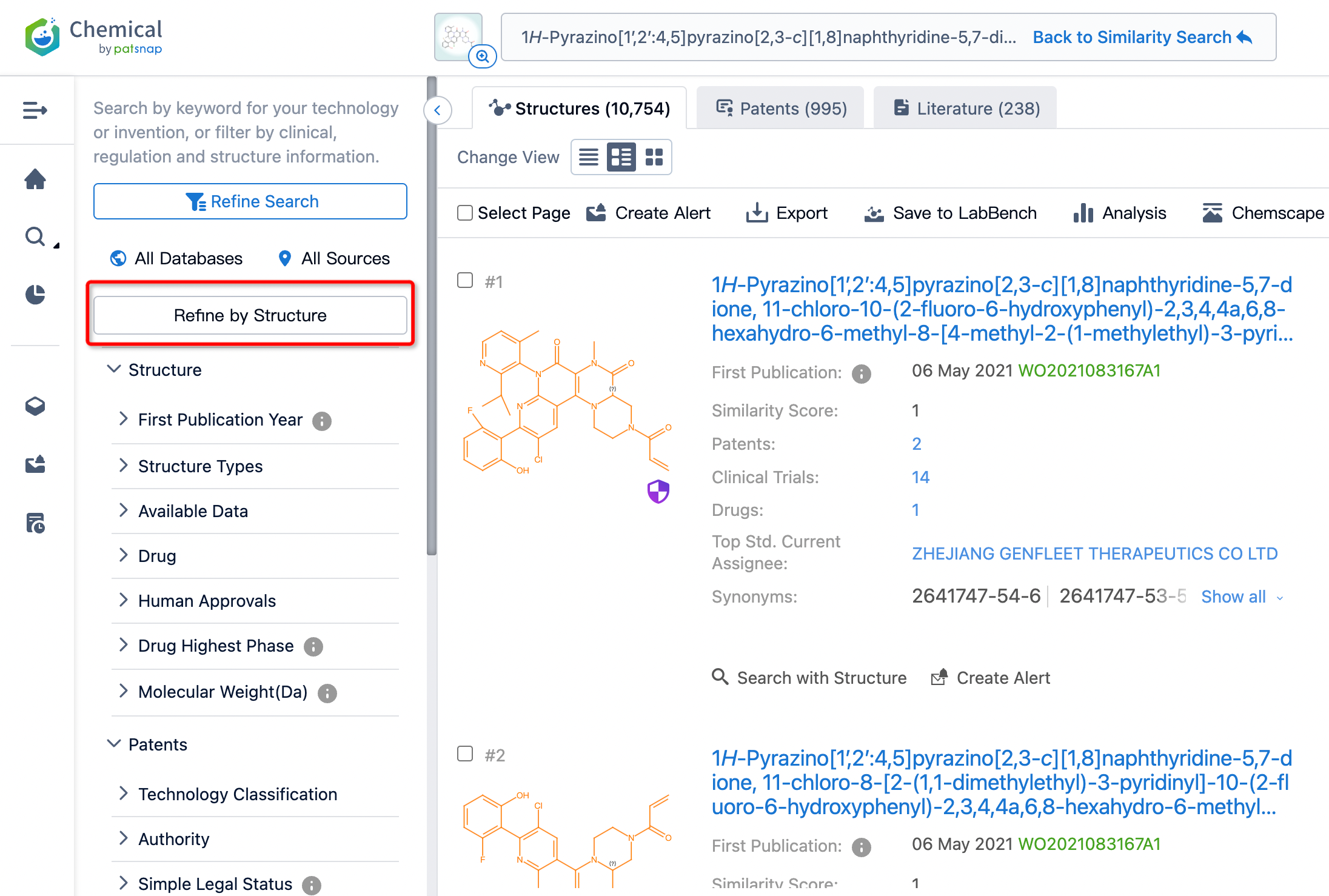
From the results, the similarity search yielded 995 relevant patent documents, which are too numerous for convenient review. To refine our search further, we can perform a secondary search by refine by structure filtering (using the core structure of Fulzerasib as a substructure for further search). In addition, depending on actual requirements, we can construct queries for a secondary search, filter patent documents and other fields, or sort search results based on technology or invention keywords, structural parameters, pharmaceutical information, etc.
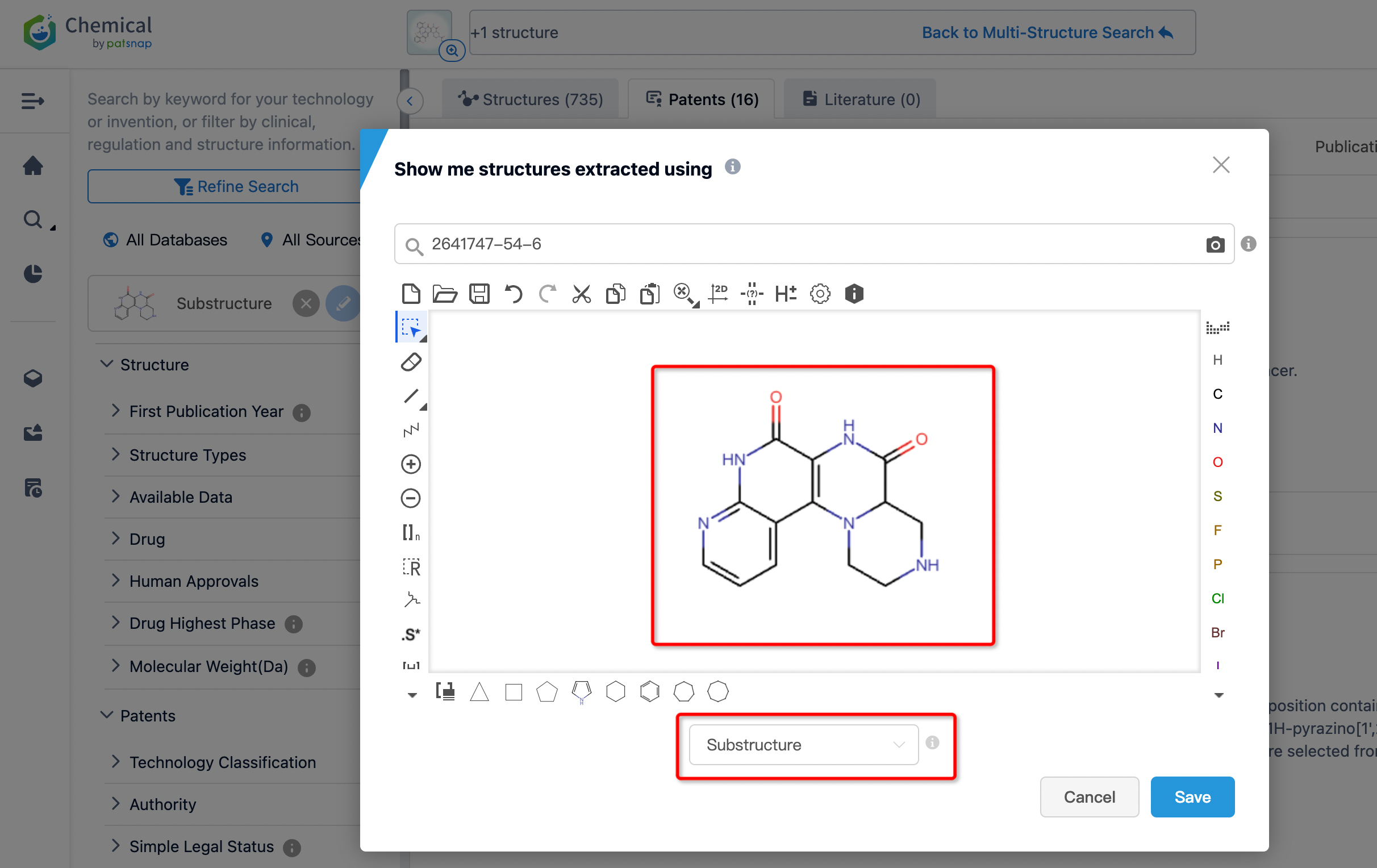
Following a second search, the number of patents was reduced to 16. From reviewing the mentioned patents, it has been observed that the main companies involved with the structure related to Fulzerasib include Genfleet Therapeutics (originating institution), Zhejiang JinFang Pharmaceutical (research institution), Zhejiang Hisun Pharmaceutical, Shanghai Ang Rui Pharmaceutical, Shanghai Jiyu Medical, Jiangxi Jemincare Group, and Shanghai Lingda Biological. The patents applied for by Shanghai Jiyu Medical and Jiangxi Jemincare Group, with application number 202010407719.2, have already been granted (grant publication number CN113321654B, grant date May 3, 2022). Similarly, Shanghai Lingda Biological’s patent, application number 202010747468.2, has also been granted (grant publication number CN112300194B, grant date January 14, 2022), and they licensed their granted patent to Genfleet Therapeutics on February 4, 2024.
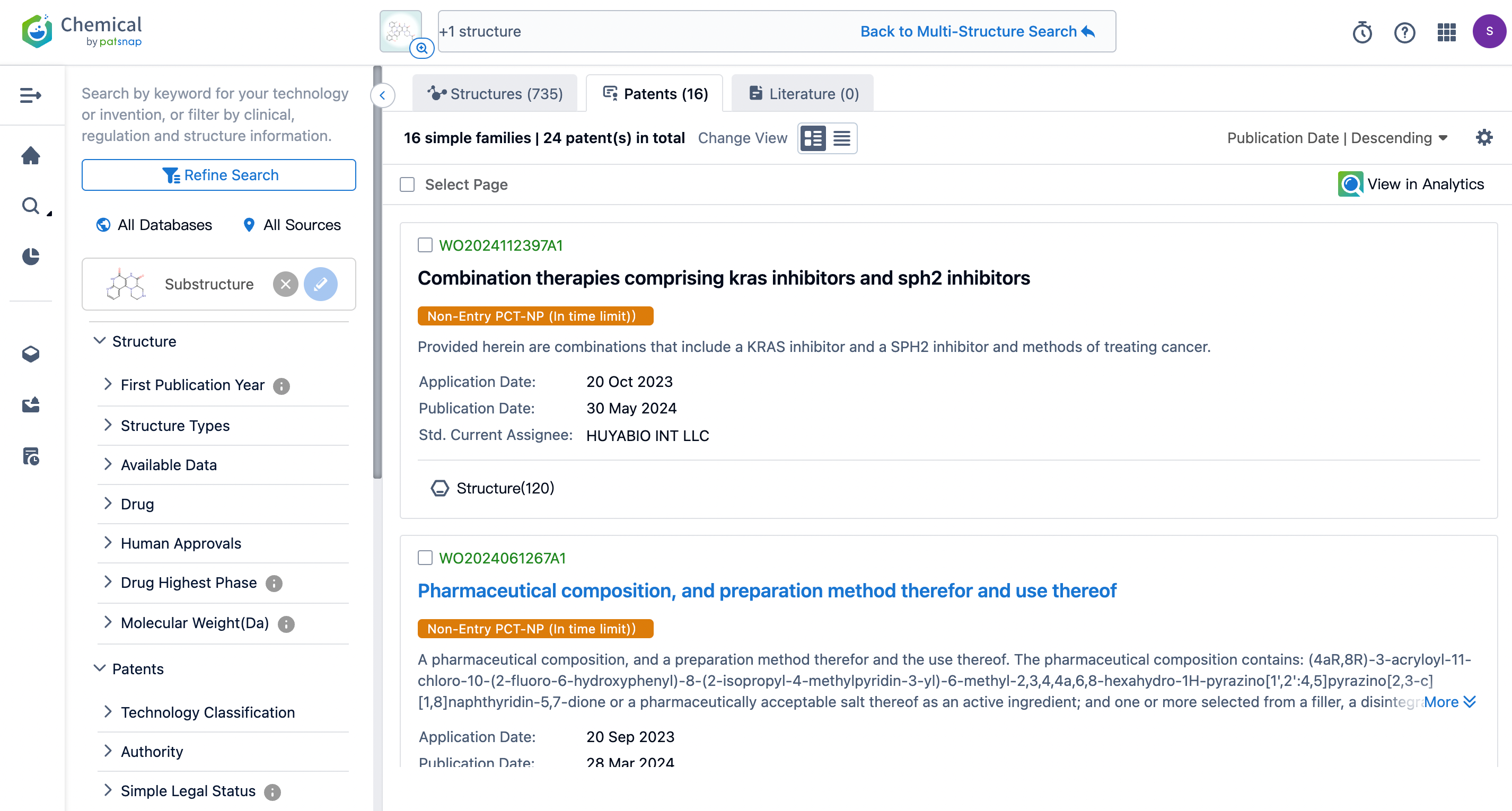
The pursuit of significant enhancements in drug efficacy through structural modifications is a current trend in pharmaceutical R&D and a crucial means to achieve higher market returns.
The combined use of the Synapse database and Patsnap Chemical provides strong support for the search and development processes in chemical pharmaceuticals. These databases integrate entities such as diseases, drugs, targets, institutions, and their relational data, offering a one-stop service for the retrieval of public information like patents, academic papers, clinical trials, and news. Through these databases, researchers can rapidly access critical information about drug development progress, clinical trial outcomes, and market transactions, which aids in strategizing drug development directions, patent layouts, and market strategies.
Particularly in the development of Me-better drugs, Patsnap Chemical offers in-depth services for drug development, patent analysis, and risk assessment. Researchers can utilize functions such as structural formula retrieval, compound information queries, and patent layout analysis to gain a comprehensive understanding of a drug’s innovation points and market potential. This enables more informed decision-making in the early stages of drug development.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.