Synapse Simplified: How to Find Allopurinol Information
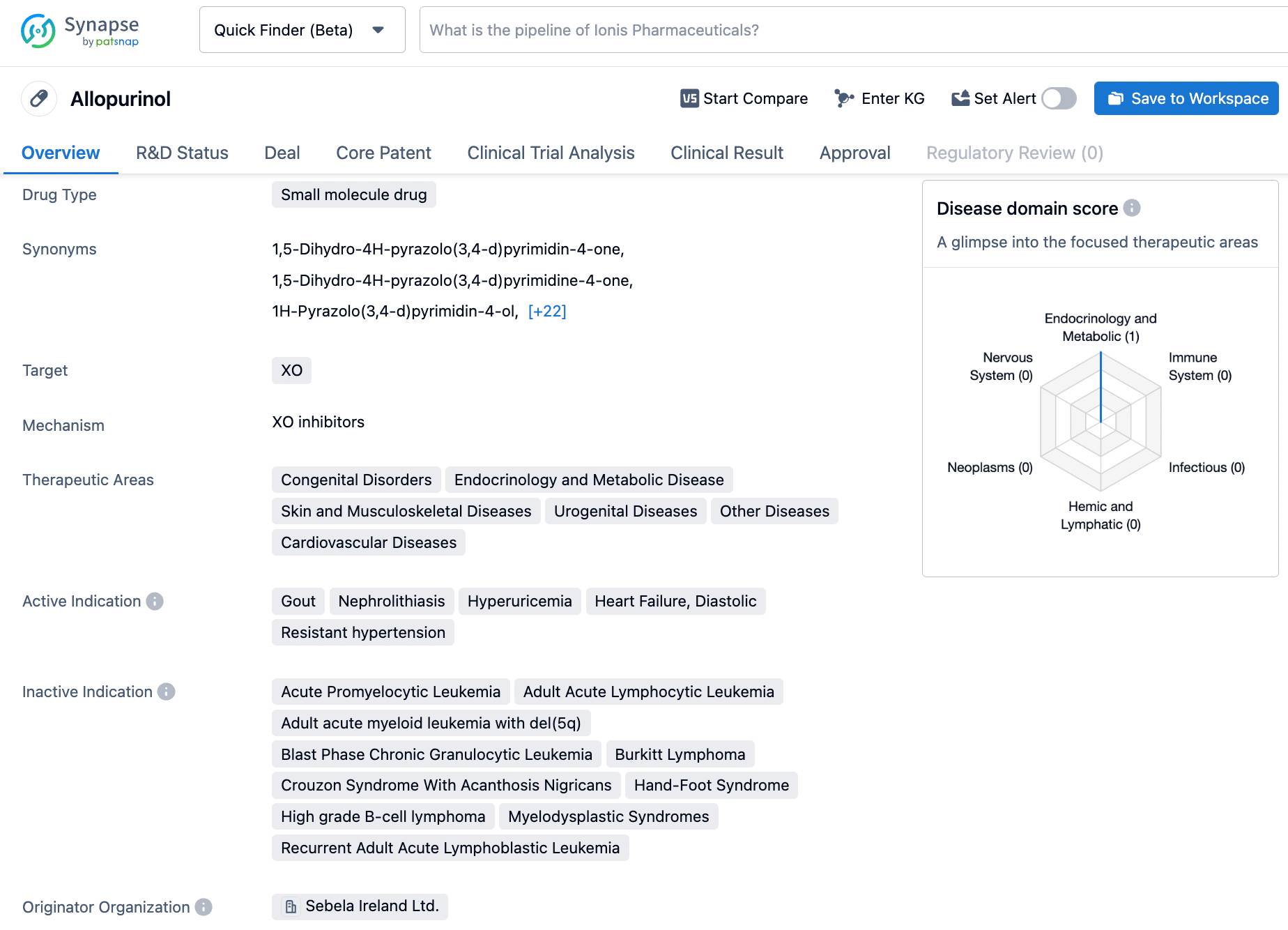
Allopurinol, marketed under the trade name Zyloprim®, is a xanthine oxidase inhibitor medication that is primarily utilized to lower urinary and serum uric acid levels in patients suffering from gout, recurrent calcium oxalate calculi, and certain malignancies. The mechanism of action of this medication involves inhibiting the enzyme xanthine oxidase. Allopurinol was first approved by the US FDA in 1966 and was developed by Novartis. Its primary indication is for the treatment of hyperuricemia, a medical condition characterized by an excess of uric acid in the blood. Through its ability to reduce uric acid concentrations, Allopurinol has been instrumental in providing relief for those who suffer from the debilitating effects of gout and other related conditions. Click on the image below to begin the exploration journey of Allopurinol through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!