First Patient Treated in Aegle Therapeutics' Phase 1/2a AGLE-102™ Trial for Dystrophic Epidermolysis Bullosa
Aegle Therapeutics Corp., a clinical-stage biopharmaceutical firm focused on creating innovative extracellular vesicle treatments for immune and inflammatory-related skin conditions, reported that the initial patient has received a dose in a Phase 1/2a clinical trial investigating AGLE-102™ as a remedy for DEB, a rare pediatric skin blistering disorder.
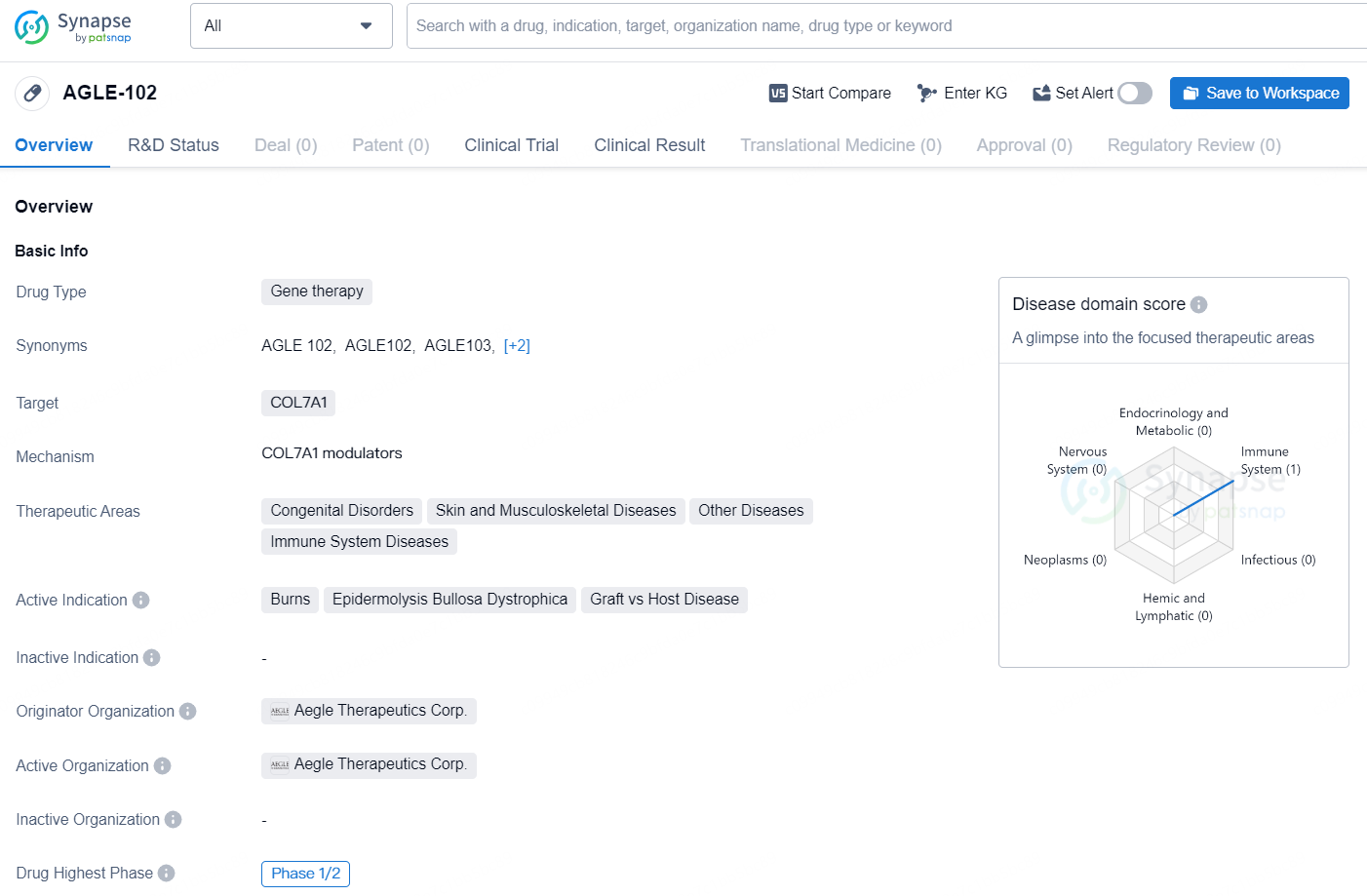
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
AGLE-102 is an experimental treatment that consists of extracellular vesicles derived from allogeneic stem cells through Aegle’s specialized techniques. These vesicles are a native mixture of cell-origin nanoparticles containing active biomolecules such as proteins and nucleic acids, which possess anti-inflammatory, immunomodulatory, and tissue regenerative properties.
"We are excited to begin the Phase 1/2a clinical trial for DEB, a challenging condition characterized by chronic blister formation and heightened inflammation. Aegle is dedicated to advancing novel treatments to meet the ongoing unmet medical needs of this patient group," stated Shelley Hartman, Aegle’s CEO.
Evangelos Badiavas, MD, PhD, Co-Founder and Chief Scientific Officer, explained, "AGLE-102 aims to replicate the body's natural healing processes by delivering proteins, like collagen 7, and other significant biomolecules such as nucleic acids. These components work by reducing inflammation, modulating the immune response, and encouraging diseased cells to produce their own collagen 7. This multifaceted approach makes AGLE-102 a unique therapeutic option for DEB."
The Phase I/2a study, titled "A Safety Study of the Administration of Mesenchymal Stem Cell Extracellular Vesicles in the Treatment of Dystrophic Epidermolysis Bullosa Wounds," is a forward-looking, non-randomized, multi-center trial set to assess the safety and efficacy of multiple AGLE-102 doses in treating chronic wounds.
AGLE-102 comprises native nanoparticles known as extracellular vesicles, which are secreted by allogeneic mesenchymal stem cells. These vesicles encompass complex arrays of active biomolecules that can elicit a broad range of responses in target cells. AGLE-102 is undergoing evaluation in two Phase 1/2a trials for DEB and severe burns.
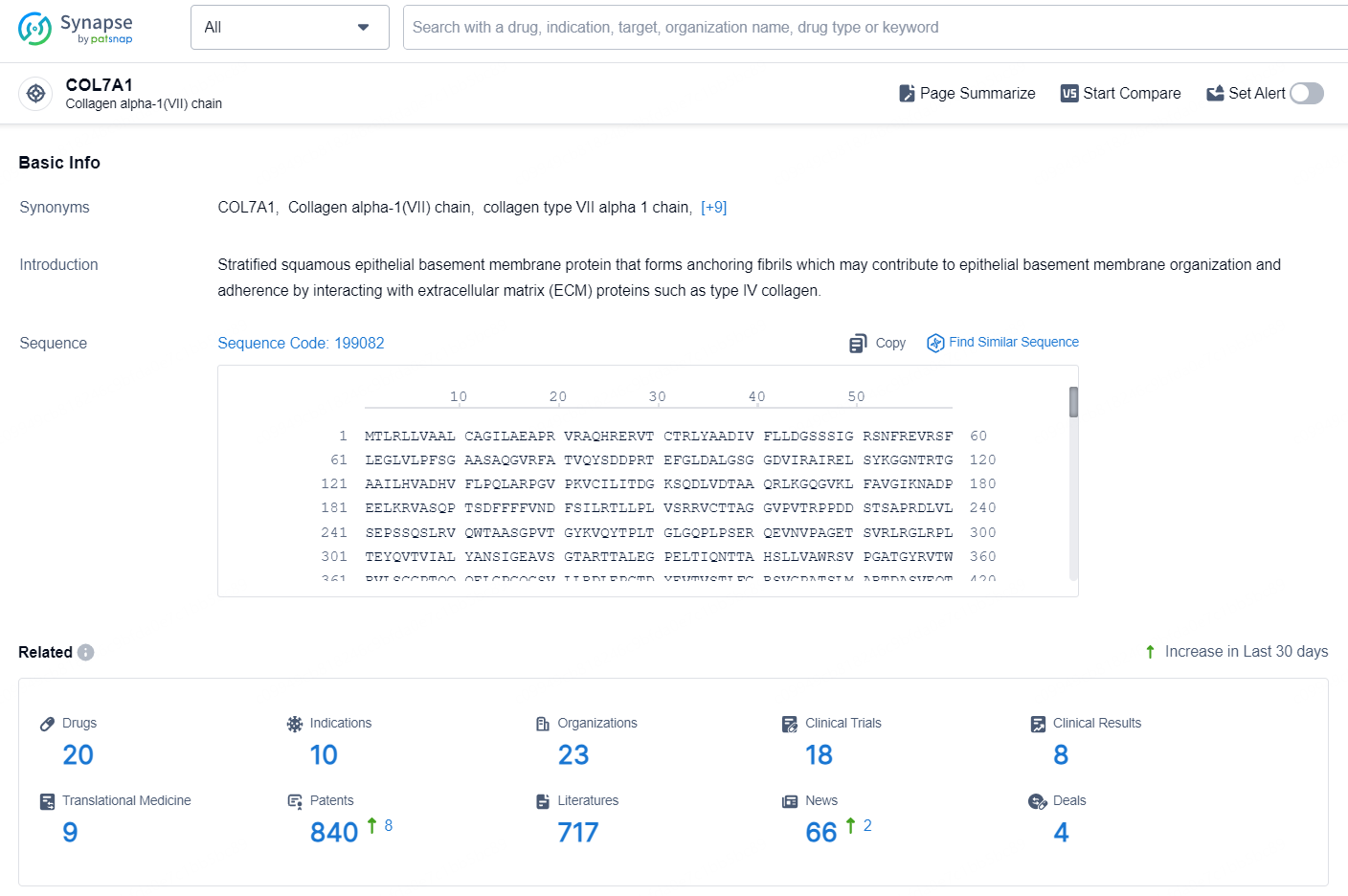
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 20, 2024, there are 20 investigational drugs for the COL7A1 target, including 10 indications, 23 R&D institutions involved, with related clinical trials reaching 18, and as many as 840 patents.
AGLE-102 targets the COL7A1 gene and shows promise in treating a range of congenital and immune system disorders. As of the latest information, the drug is in the Phase 1/2 stage of development, and further clinical trials will be necessary to assess its overall therapeutic potential.