LAVA Therapeutics Partners with Merck & Co. for Potential Combination of LAVA-1207 and KEYTRUDA®
LAVA Therapeutics N.V. has revealed the establishment of a pact for a clinical study and provision of materials with Merck & Co., Inc., based in Rahway, NJ, USA. The purpose of this collaboration is to scrutinize the effects of Merck's immunotherapy, KEYTRUDA® (pembrolizumab), a prominent anti-PD-1 drug, when administered in unison with LAVA's innovative Gammabody®, LAVA-1207. LAVA-1207 has been meticulously engineered to hone in on the prostate-specific membrane antigen (PSMA), enabling the targeted and effective eradication of tumor cells exhibiting PSMA, within the patient subgroup afflicted with metastatic castration-resistant prostate cancer that has demonstrated resistance to standard treatments.
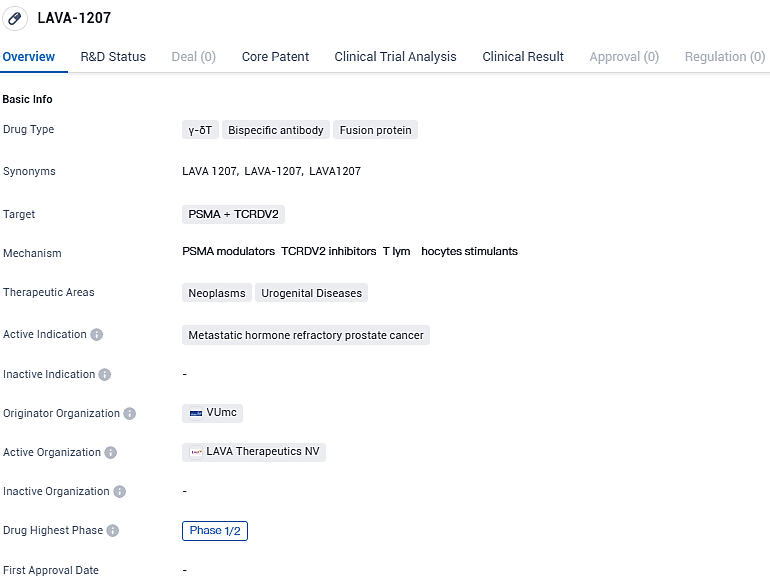
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In accordance with the partnership conditions, the pharmaceutical entity Merck & Co., Inc., based in Rahway, NJ, USA, has committed to supply pembrolizumab to support the upcoming stages of dose escalation and broadening in LAVA's active Phase 1/2a clinical trial of LAVA-1207. Plans are set to initiate the trial's combined therapy arm within the initial six months of 2024. The process of participant enrollment and progressive dosage increases will persist in LAVA-1207's single-agent arm and the arm testing the effects of interleukin-2.
"We are delighted at the prospect of teaming up with Merck & Co., Inc., Rahway, NJ, USA to advance our understanding of LAVA-1207's therapeutic potential, particularly in its combined use with KEYTRUDA®," expressed Stephen Hurly, the head and chief officer of LAVA.
Stephen Hurly further elaborated, "To this point, LAVA-1207 has exhibited an encouraging safety landscape and initial indicators of its capacity to counteract tumor growth. Given the difficulties faced in utilizing immune checkpoint treatments for prostate cancer, we harbor optimism for the combined impact of our therapeutic offerings in achieving significant clinical results."
Hans van der Vliet, M.D., Ph.D., the principal scientific figurehead at LAVA, remarked, "The generally suppressive nature of the tumor environment observed within a majority of patients with metastatic castration-resistant prostate cancer (mCRPC) has led to suboptimal tumor-fighting effects from immune checkpoint inhibitors. With the combined deployment of LAVA-1207 and pembrolizumab, we aim to assess the activation potential of Vγ9Vδ2 T cells in aggressively targeting prostate cancer, while potentially enhancing the immune system's wide-spectrum tumor response, particularly when PD-1 is inhibited through pembrolizumab."
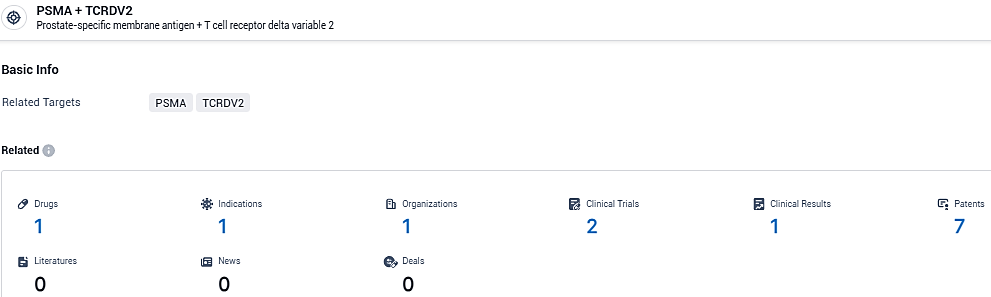
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 30, 2024, there are 1 investigational drugs for the PSMA and TCRDV2 target, including 1 indications, 1 R&D institutions involved, with related clinical trials reaching 2, and as many as 7 patents.
LAVA-1207 is a promising drug in the field of biomedicine that targets PSMA and TCRDV2. It aims to treat metastatic hormone refractory prostate cancer, a challenging condition that has limited treatment options. Further research and development will be necessary to assess the drug's potential and bring it closer to commercial available.