Formycon and Fresenius Kabi Reach Agreement on Ustekinumab Biosimilar FYB202 for European and Canadian Markets
Formycon AG alongside its marketing associate Fresenius Kabi made public the culmination of a resolution accord with Johnson & Johnson regarding the market introduction of FYB202, a biosimilar counterpart to Stelara® (ustekinumab), targeting the European and Canadian territories. The parties have concurred upon maintaining the particulars of the accord under wraps.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Nicola Mikulcik, the Executive for Commercial Strategy at Formycon AG, has stated: "We are dedicated to transforming the healthcare experience for individuals by offering treatments that are not only trustworthy and of superior quality but are also economically feasible. With the U.S. resolution in place, we've taken yet another pivotal stride. This reinforces the collaborative pursuit shared by Formycon and Fresenius Kabi to ensure the global availability of FYB202."
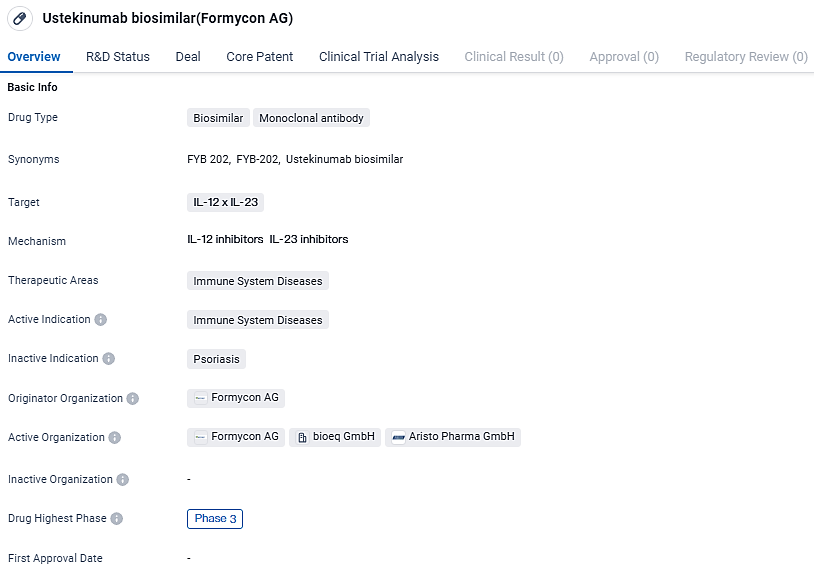
FYB202 is a biologic that acts as a monoclonal antibody, interacting specifically with cytokines interleukin-12 and interleukin 23. It is prescribed for a range of severe conditions that are immune-mediated. Stelara® is recognized for managing conditions from moderate to severe plaque psoriasis to Crohn’s disease, ulcerative colitis, and active psoriatic arthritis.
In terms of regulatory progress, the European Medicines Agency commenced its review of the marketing authorization application for FYB202 in September 2023, followed by the U.S. Food and Drug Administration in November 2023. Earlier in February 2023, Formycon and Fresenius Kabi came to a licensing agreement on a worldwide scale for the biosimilar candidate of ustekinumab, FYB202. Post-approval, Fresenius Kabi will take on the marketing responsibilities for FYB202 in essential international territories.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
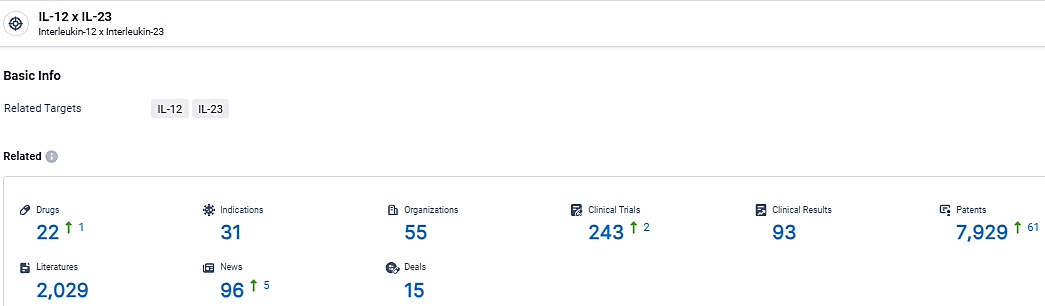
According to the data provided by the Synapse Database, As of March 19 2024, there are 22 investigational drugs for the IL-12 and IL-23 target, including 31 indications, 55 R&D institutions involved, with related clinical trials reaching 243, and as many as 7929 patents.
Ustekinumab biosimilar is a monoclonal antibody drug that falls under the category of biosimilars. It specifically targets the IL-12 x IL-23 proteins, which are involved in immune system regulation. The drug is primarily intended for the treatment of immune system diseases.